On page 3360 of the 27 November 2014 issue, a sentence is omitted from the last footnote of Table 1. The footnote should have read, “†Enrollment as of June 2014. Of enrolled patients, 173 have early-stage disease.” Also on page 3360, there is an error in Figure 3E. An incorrect version of the panel was included. The corrected Table 2 footnote and Figure 3 are shown below. The errors have been corrected in the online version, which now differs from the print version.
Prospective noncontrolled response-adapted studies in adult early-stage (I-II) HL
| Trial . | Patients . | Treatment . | Number . | Interim PET+ . | PPV . | NPV . | Survival . |
|---|---|---|---|---|---|---|---|
| Le Roux et al, 201128 | Stages I-IV | ABVD × 4 (FDG-PET): I/II nonbulky: PET− and/or CR on CT IFRT; PET+ SCT II bulky/III/IV: PET− ABVD × 4; PET+ SCT | 90 (45 stage I/II) | 34% (all patients) | 16% (all patients) | 95% (all patients) | NA |
| Dann et al, 201340 | Stage I-IIA-B nonbulky | ABVD × 2 (FDG-PET): favorable: PET− INRT; PET+ ABVD × 2 + INRT (PET 4)* Unfavorable: PET− ABVD × 2 + INRT; PET+ ABVD × 4 + INRT (PET 4)* | 350/350† | 13% | 26% | 93% | 2-y PFS 94% |
| CALGB 50604 (NCT01132807) | Stage I/IIA-B nonbulky | ABVD × 2 (FDG-PET): PET− ABVD × 2 PET+ BEACOPP-escalated × 2 + 30Gy IFRT | 160/160 | Accrual completed February 2013; preliminary results expected 2015 | |||
| CALGB 50801 (NCT01118026) | Stage I/IIA-B bulky | ABVD × 2 (FDG-PET): PET− ABVD × 4 PET+ BEACOPP-escalated × 4 + 30Gy IFRT | 53/123† | NA | |||
| Trial . | Patients . | Treatment . | Number . | Interim PET+ . | PPV . | NPV . | Survival . |
|---|---|---|---|---|---|---|---|
| Le Roux et al, 201128 | Stages I-IV | ABVD × 4 (FDG-PET): I/II nonbulky: PET− and/or CR on CT IFRT; PET+ SCT II bulky/III/IV: PET− ABVD × 4; PET+ SCT | 90 (45 stage I/II) | 34% (all patients) | 16% (all patients) | 95% (all patients) | NA |
| Dann et al, 201340 | Stage I-IIA-B nonbulky | ABVD × 2 (FDG-PET): favorable: PET− INRT; PET+ ABVD × 2 + INRT (PET 4)* Unfavorable: PET− ABVD × 2 + INRT; PET+ ABVD × 4 + INRT (PET 4)* | 350/350† | 13% | 26% | 93% | 2-y PFS 94% |
| CALGB 50604 (NCT01132807) | Stage I/IIA-B nonbulky | ABVD × 2 (FDG-PET): PET− ABVD × 2 PET+ BEACOPP-escalated × 2 + 30Gy IFRT | 160/160 | Accrual completed February 2013; preliminary results expected 2015 | |||
| CALGB 50801 (NCT01118026) | Stage I/IIA-B bulky | ABVD × 2 (FDG-PET): PET− ABVD × 4 PET+ BEACOPP-escalated × 4 + 30Gy IFRT | 53/123† | NA | |||
SCT, stem cell transplantation; and NA, not available.
Biopsy done if PET-4 is positive; patients receive same therapy as PET-4− for negative biopsy and salvage therapy for positive PET-4 biopsy.
Enrollment as of June 2014. Of enrolled patients, 173 have early-stage disease.
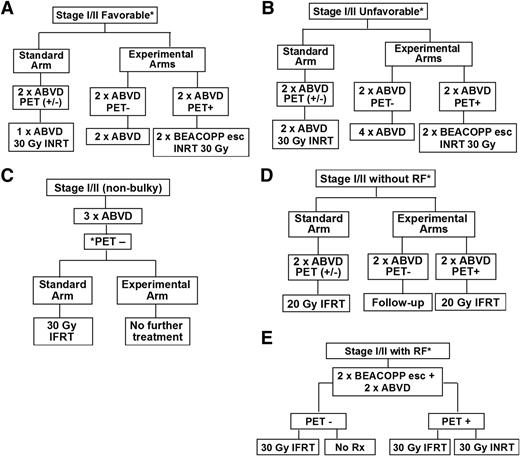
Clinical trial designs of recently completed and ongoing phase 3 randomized studies of response-adapted therapy for adult early-stage HL. (A) EORTC/LYSA/FIL H10F study. *None of the following present: large mediastinal mass, age ≥50 years, high ESR, or 4 or more areas. (B) EORTC/LYSA/FIL H10U study. *Any of the following present: large mediastinal mass, age ≥50 years, high ESR, and/or 4 or more areas. (C) UK-led RAPID study; all PET-3+ patients received a 4th cycle of ABVD followed by 30 Gy of IFRT. (D) GHSG HD16 favorable trial. *None of the following present: large mediastinal mass, extranodal disease, high ESR, or 3 or more areas. (E) GHSG HD17 unfavorable trial. *Any of the following present: large mediastinal mass, extranodal disease, high ESR, and/or 3 or more areas. High ESR for all of above defined as: >50 mm without B symptoms or ESR <30 mm with B symptoms. esc indicates escalated; ESR, erythrocyte sedimentation rate; LYSA, Lymphoma Group and the Lymphoma Study Association; FIL, Fondazione Italiana Linfomi; and pts, patients.
Clinical trial designs of recently completed and ongoing phase 3 randomized studies of response-adapted therapy for adult early-stage HL. (A) EORTC/LYSA/FIL H10F study. *None of the following present: large mediastinal mass, age ≥50 years, high ESR, or 4 or more areas. (B) EORTC/LYSA/FIL H10U study. *Any of the following present: large mediastinal mass, age ≥50 years, high ESR, and/or 4 or more areas. (C) UK-led RAPID study; all PET-3+ patients received a 4th cycle of ABVD followed by 30 Gy of IFRT. (D) GHSG HD16 favorable trial. *None of the following present: large mediastinal mass, extranodal disease, high ESR, or 3 or more areas. (E) GHSG HD17 unfavorable trial. *Any of the following present: large mediastinal mass, extranodal disease, high ESR, and/or 3 or more areas. High ESR for all of above defined as: >50 mm without B symptoms or ESR <30 mm with B symptoms. esc indicates escalated; ESR, erythrocyte sedimentation rate; LYSA, Lymphoma Group and the Lymphoma Study Association; FIL, Fondazione Italiana Linfomi; and pts, patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal