Key Points
Transcriptome and functional analyses reveal accelerated T-cell aging in ICL.
Dampening of TCR signaling in ICL relies on DUSP4 overexpression.
Abstract
Idiopathic CD4 lymphopenia (ICL) is a rare heterogeneous immunological syndrome of unclear etiology. ICL predisposes patients to severe opportunistic infections and frequently leads to poor vaccination effectiveness. Chronic immune activation, expansion of memory T cells, and impaired T-cell receptor (TCR) signaling have been reported in ICL, but the mechanistic and causative links remain unclear. We show that late-differentiated T cells in 20 patients with ICL displayed defective TCR responses and aging markers similar to those found in T cells from elderly subjects. Intrinsic T-cell defects were caused by increased expression of dual-specific phosphatase 4 (DUSP4). Normalization of DUSP4 expression using a specific siRNA improved CD4+ T-cell activity in ICL, as this restored TCR-induced extracellular signal-regulated kinase activation and increased the expression of the costimulatory molecules CD27 and CD40L. Conversely, repeated TCR stimulation led to defective signaling and DUSP4 overexpression in control CD4+ T cells. This was associated with gradual acquisition of a memory phenotype and was curtailed by DUSP4 silencing. These findings identify a premature T-cell senescence in ICL that might be caused by chronic T-cell activation and a consequential DUSP4-dependent dampening of TCR signaling.
Introduction
Idiopathic CD4 lymphopenia (ICL) is a rare disorder characterized by an absolute CD4+ T-cell count lower than 300/mm3 or lower than 20% of the total T cells on more than 1 cell count in the absence of infection with HIV-1/2 or human T-lymphotropic virus 1/2 or other identified causes of lymphopenia.1,2 ICL is most frequently diagnosed in adults, although pediatric cases have been reported.2-5 Patients may show opportunistic infections as a result of a profound deficiency in cell-mediated immune responses.2,6,7 Although the molecular mechanisms underlying this heterogeneous syndrome are still unclear, loss-of-function mutations have been described for genes encoding various regulators of T-cell receptor (TCR) diversity and signaling.3,4,8-10 In line with the weak adaptive immunity in ICL, antigen-induced lymphoproliferation, cytokine production, and vaccination responses are diminished.3,11,12
ICL might result from defective thymic production or output of T cells, exacerbated peripheral consumption, or any combination of these.4-6,12-18 Cell-intrinsic or cell-extrinsic factors have been identified in ICL that may participate in the perturbation of CD4+ T-cell homeostasis. These include altered responses to homeostatic cytokines, such as interleukin-2 (IL-2), IL-7, and CXC chemokine ligand 12,12,15,16 and an enhanced susceptibility of ICL-derived T cells to undergo apoptosis.17,18 Activation and turnover markers are higher in ICL CD4+ T cells, and the expression levels of these markers inversely correlate with blood CD4+ T-cell counts.6 Abnormal T-cell activation was also associated with high levels of microbial translocation products in ICL plasma.19 Thus, persistent stimulation by an unidentified pathogen may be one cause of the chronic immune activation and preferential loss of naive T cells in ICL.11
Mechanistic studies have pointed to defective TCR signal transduction in ICL. In one case, low lymphocyte-specific protein tyrosine kinase (Lck)/p56 activity accounted for weak CD4+ T-cell proliferation in response to CD3/TCR stimulation.20 In addition, a dominant-negative missense mutation has been identified in 1 patient that targets the signaling adaptor protein Unc119 required for Lck transport and activation, and thus for TCR-triggered responses.8 X-linked mutations in the magnesium transporter gene 1 (MAGT1) were also described in 2 related patients, suggesting a role for Mg2+ as an intracellular second messenger that couples TCR activation to intracellular effectors.3 These findings raise the possibility that persistent T-cell activation in ICL leads to dampened TCR-mediated signaling and proliferation.
To gain further insight into the functional pathways implicated in ICL pathophysiology, we compared the gene expression profiles of CD4+ T cells sorted from the blood of patients with ICL and control groups of age-matched healthy subjects and patients with CD4+ T-cell lymphopenia secondary to sarcoidosis (SARC).21 These arrays enabled us to identify an ICL-related genetic signature with predominant involvement of 2 gene groups: one related to TCR response and the other strikingly associated with T-cell aging. Among the first group, expression of the dual-specific phosphatase 4 (DUSP4), which negatively regulates mitogen-activated protein kinase (MAPK) activation, was increased, similar in this respect to CD4+ T cells from elderly subjects. Functional studies further linked this overexpression to accelerated T-cell senescence in ICL.
Methods
Subjects
Twenty adult patients who met the definition of ICL established by the Centers for Disease Control and Prevention1 were recruited at the Cochin Hospital and Centre d’Infectiologie Necker-Pasteur in Paris, France. Descriptions of the healthy, SARC, elderly, and ICL groups are provided in Table 1, supplemental Table 1, and the supplemental Methods, available on the Blood Web site. The study was approved by the Comité de Protection des Personnes Île-De-France-I (number 2012-A00172-41).
Clinical and immunological characteristics of study participants
| Characteristics . | Healthy (n = 28) . | Elderly (n = 6) . | SARC (n = 8) . | ICL (n = 20) . |
|---|---|---|---|---|
| Age, years | 43.5 (24-60) | 91.5 (87-99) | 47.5 (31-76) | 51.5 (24-76) |
| Sex (male:female) | 11:17 | 1:5 | 1:7 | 10:10 |
| CD4+ T-cell count, cells/mm3 | 774 (510-1037) | 478 (330-687)* | 393 (200-723)* | 100 (22-278)*** |
| CD4+ cells, % | 46 (37-55) | 47.5 (34.6-57.2) | 34.5 (20-59) | 23 (3-62)** |
| CD8+ T-cell count, cells/mm3 | 437 (258-615) | 261 (164-375) | 372 (175-393) | 112 (15-691)* |
| CD8+ cells, % | 27 (18-35) | 25 (16-30) | 24 (15-25) | 20 (9-77) |
| CD4:CD8 ratio | 1.7 (0.8-4) | 1.4 (0.8-1.8) | 1.3 (0.6-2.4) | 1 (0.05-2.5)* |
| CD19+ B-cell count, cells/mm3 | 239 (137-341) | 112 (52-278)* | 234 (112-378) | 71 (6-547)*** |
| CD19+ cells, % | 16 (10-21) | 14 (7-20) | 18 (12-26) | 19 (1.5-46) |
| Immunoglobulins (g/L) | ||||
| IgG | 10.9 (6.8-15) | ND | 12.7 (10.9-16.6) | 9.4 (6.2-12.2) |
| IgA | 2.25 (0.75-3.75) | ND | 2.62 (2.58-2.99) | 1.17 (0.4-3.3) |
| IgM | 1.3 (0.40-2.20) | ND | 0.75 (0.66-1.75) | 0.65 (0.37-2.73) |
| Opportunistic infections | ||||
| Human papillomavirus | — | — | — | 6 |
| Varicella zoster virus reactivation | — | 1 | — | 2 |
| Cryptococcus neoformans | — | — | — | 2† |
| Pneumocystis jiroveci | — | — | — | 2 |
| Mycobacterium avium complex | — | — | — | 1† |
| Asymptomatic | — | 5 | — | 8 |
| Dysimmune diseases | ||||
| Autoimmune hemolytic anemia | — | — | — | 1 |
| Systemic lupus erythematosus | — | — | — | 1 |
| Vitiligo | — | — | — | 1 |
| Crohn’s disease | — | — | — | 1 |
| Cytomegalovirus status | ||||
| Serology-positive (IgM− IgG+) | ND | ND | ND | 8 |
| PCR-positive | ND | ND | ND | 0 |
| Characteristics . | Healthy (n = 28) . | Elderly (n = 6) . | SARC (n = 8) . | ICL (n = 20) . |
|---|---|---|---|---|
| Age, years | 43.5 (24-60) | 91.5 (87-99) | 47.5 (31-76) | 51.5 (24-76) |
| Sex (male:female) | 11:17 | 1:5 | 1:7 | 10:10 |
| CD4+ T-cell count, cells/mm3 | 774 (510-1037) | 478 (330-687)* | 393 (200-723)* | 100 (22-278)*** |
| CD4+ cells, % | 46 (37-55) | 47.5 (34.6-57.2) | 34.5 (20-59) | 23 (3-62)** |
| CD8+ T-cell count, cells/mm3 | 437 (258-615) | 261 (164-375) | 372 (175-393) | 112 (15-691)* |
| CD8+ cells, % | 27 (18-35) | 25 (16-30) | 24 (15-25) | 20 (9-77) |
| CD4:CD8 ratio | 1.7 (0.8-4) | 1.4 (0.8-1.8) | 1.3 (0.6-2.4) | 1 (0.05-2.5)* |
| CD19+ B-cell count, cells/mm3 | 239 (137-341) | 112 (52-278)* | 234 (112-378) | 71 (6-547)*** |
| CD19+ cells, % | 16 (10-21) | 14 (7-20) | 18 (12-26) | 19 (1.5-46) |
| Immunoglobulins (g/L) | ||||
| IgG | 10.9 (6.8-15) | ND | 12.7 (10.9-16.6) | 9.4 (6.2-12.2) |
| IgA | 2.25 (0.75-3.75) | ND | 2.62 (2.58-2.99) | 1.17 (0.4-3.3) |
| IgM | 1.3 (0.40-2.20) | ND | 0.75 (0.66-1.75) | 0.65 (0.37-2.73) |
| Opportunistic infections | ||||
| Human papillomavirus | — | — | — | 6 |
| Varicella zoster virus reactivation | — | 1 | — | 2 |
| Cryptococcus neoformans | — | — | — | 2† |
| Pneumocystis jiroveci | — | — | — | 2 |
| Mycobacterium avium complex | — | — | — | 1† |
| Asymptomatic | — | 5 | — | 8 |
| Dysimmune diseases | ||||
| Autoimmune hemolytic anemia | — | — | — | 1 |
| Systemic lupus erythematosus | — | — | — | 1 |
| Vitiligo | — | — | — | 1 |
| Crohn’s disease | — | — | — | 1 |
| Cytomegalovirus status | ||||
| Serology-positive (IgM− IgG+) | ND | ND | ND | 8 |
| PCR-positive | ND | ND | ND | 0 |
Data are medians (ranges). All P values are compared with healthy individuals (as determined using the Mann-Whitney U test).
ND, not determined.
P < .05.
P < .005.
P < .0005.
One patient with Cryptococcus neoformans and Mycobacterium avium complex coinfection.
Sample processing
Whole-blood phenotyping of T-cell subsets was performed as described.22 Isolation, cryopreservation, thawing, and staining of CD4+ T cells and peripheral blood mononuclear cells (PBMCs) were performed as described,23,24 using monoclonal antibodies detailed in the supplemental Methods. Carboxyfluorescein diacetate succinimidyl ester (CFSE)-loaded PBMCs or CD4+ T cells were either left untreated or stimulated once, twice, or 3 times on anti-CD3/anti-CD28 antibody (Ab)-coated plates for 5 days. Data were collected on a Fortessa flow cytometer.
Microarray analysis
Total RNA was isolated from CD4+ T-cell pellets obtained from healthy, SARC, and ICL subjects, using the miRNAeasy mini kit (Qiagen). Microarray analysis was performed using Affymetrix Human Exon 1.0 ST, according to the manufacturer’s recommendations. Data set analysis and visualization were done using EASANA, which is based on GenoSplice FAST DB annotations.25,26 Array data are available at Gene Expression Omnibus (accession number GSE56998). For details, see supplemental Methods.
Methods for polymerase chain reaction (PCR), DUSP4 silencing, immunoblot, enzyme-linked immunosorbent assay, telomerase activity, telomere length, and chemotaxis assays, as well as statistical analyses, are described in supplemental Methods.
Results
Expansion of circulating terminally differentiated effector memory T cells in ICL
We analyzed 20 patients with ICL (Table 1). CD4+ T-cell counts were significantly lower in patients with ICL than in age-matched healthy subjects, and to a lesser extent, these counts were lower than in untreated and uninfected patients with lymphopenia secondary to SARC (supplemental Table 1). CD8+ T-cell counts were lower in patients with ICL than in healthy individuals, although at a lower level of significance. B-cell counts were also significantly lower in patients with ICL, although this did not correspond to lower immunoglobulin (Ig) levels in patient sera. Our cohort was clinically heterogeneous, as 40% of the patients were asymptomatic and 60% presented with opportunistic infections typically observed in CD4+ T-cell deficiencies such as AIDS.27 Four patients with ICL suffered from dysimmune diseases.
Whole-blood immunophenotyping indicated that circulating CD4+ and CD8+ T-cell lymphopenia in ICL predominantly affected naive cells (Table 2; supplemental Tables 2 and 3). Analyses of the memory T-cell compartment defined by CD45RA and CCR7 expression levels showed that the terminally differentiated effector memory (TEMRA; CD45RA+CCR7−) subpopulation was expanded in patients with ICL. Contraction of the naive subset and inflation of the TEMRA compartment both correlated with the degree of T-cell lymphopenia (supplemental Figure 1 and Table 3). Impaired T-cell expression of CXCR4, which binds the chemokine CXC chemokine ligand 12, was reported for 6 patients with ICL.12 We assessed whether CXCR4 dysfunction extended to our larger cohort (Table 2 and supplemental Figure 2). Compared with healthy and SARC T cells, we detected reduced levels of CXCR4 expression and chemotactic function in naive and activated-memory T cells from 17 of 20 patients with ICL. Low levels of CXCR4 expression correlated with the degree of T-cell lymphopenia. Therefore, patients with ICL and SARC differ in peripheral T-cell subset distribution and CXCR4 status.
Immunophenotyping of CD4+ T-cell populations
| . | Healthy (n = 28) . | Elderly (n = 6) . | SARC (n = 8) . | ICL (n = 20) . |
|---|---|---|---|---|
| % CD4+ in T CD3+ | 61.2 (44.1-71.5) | 52.8 (29.9-77.8)* | 52.3 (28.3-65.2)* | 38.7 (3.52-76.1)**,† |
| In T CD4+: | ||||
| % CCR7+ CD45RA+ (NV) | 54.9 (18.9-64.3) | 25.9 (11-37.7)*** | 41.2 (25.2-56.3)* | 27.6 (11.2-52.4)*** |
| % CCR7+ CD45RA− (CM) | 28.2 (12.3-41.4) | 19.7 (9.5-49.7) | 10.2 (5.2-29.1)** | 18.2 (5.1-41.3) |
| % CCR7− CD45RA− (EM) | 15.6 (5.2-35.5) | 23.3 (14.6-37.5)* | 20.4 (15.3-47.3)* | 30.4 (10.1-44.9)** |
| % CCR7− CD45RA+ (TEMRA) | 10.1 (5-22.3) | 31 (17.2-42.5)* | 19.6 (17.3-30.2)* | 28.5 (12.5-40.8)** |
| Correlation factor (r Spearman’s and P value) | ||||
| Percentage naive and CD4+ T-cell count | ns | 0.90 and 0.04 | ns | 0.44 and 0.049 |
| Percentage activated/memory and CD4+ T-cell count | ns | −0.90 and 0.04 | ns | −0.54 and 0.014 |
| CXCR4 in T CD4+(MFI) | ||||
| Total | 586 (189-805) | 572 (190-1231) | 500 (248-610) | 199 (47-639)***,† |
| NV | 912 (610-1010) | 927 (712-1425) | 897 (745-958) | 287 (112-314)***,† |
| CM | 294 (112-357) | 274 (97-412) | 257 (100-387) | 124 (58-220)*,† |
| EM | 151 (92-245) | 167 (102-258) | 102 (56-257) | 64 (42-112)**,† |
| TEMRA | 382 (212-478) | 427 (247-528) | 450 (312-524) | 77 (55-97)***,† |
| CXCR4 in T CD4+(%) | ||||
| Total | 61.5 (38.4-75.7) | 67 (37.2-89.3) | 60.4 (42.1-76.2) | 39.5 (12.4-65.7)**,† |
| NV | 80.2 (64.1-94-1) | 84.3 (68.2-97.4) | 77.5 (55.2-84.3) | 39.1 (12.6-48.4)***,† |
| CM | 53.2 (44.7-67.7) | 56.1 (45.3-71.3) | 49.7 (32.1-55.4) | 32.4 (22.8-48.6)*,† |
| EM | 40.7 (35.1-52.1) | 39.3 (42.1-64.8) | 42.4 (22.4-58.4) | 24.9 (11.3-28.7)**,† |
| TEMRA | 59.5 (32.4-64.4) | 66.2 (42.1-77.3) | 51.8 (33.7-64.7) | 26.4 (8.3-32.7)**,† |
| Correlation factor (r Spearman’s and P value) | ||||
| CXCR4 MFI and CD4+ T-cell count | ns | ns | ns | 0.45 and 0.039 |
| CXCR4 % and CD4+ T-cell count | ns | ns | ns | 0.46 and 0.035 |
| . | Healthy (n = 28) . | Elderly (n = 6) . | SARC (n = 8) . | ICL (n = 20) . |
|---|---|---|---|---|
| % CD4+ in T CD3+ | 61.2 (44.1-71.5) | 52.8 (29.9-77.8)* | 52.3 (28.3-65.2)* | 38.7 (3.52-76.1)**,† |
| In T CD4+: | ||||
| % CCR7+ CD45RA+ (NV) | 54.9 (18.9-64.3) | 25.9 (11-37.7)*** | 41.2 (25.2-56.3)* | 27.6 (11.2-52.4)*** |
| % CCR7+ CD45RA− (CM) | 28.2 (12.3-41.4) | 19.7 (9.5-49.7) | 10.2 (5.2-29.1)** | 18.2 (5.1-41.3) |
| % CCR7− CD45RA− (EM) | 15.6 (5.2-35.5) | 23.3 (14.6-37.5)* | 20.4 (15.3-47.3)* | 30.4 (10.1-44.9)** |
| % CCR7− CD45RA+ (TEMRA) | 10.1 (5-22.3) | 31 (17.2-42.5)* | 19.6 (17.3-30.2)* | 28.5 (12.5-40.8)** |
| Correlation factor (r Spearman’s and P value) | ||||
| Percentage naive and CD4+ T-cell count | ns | 0.90 and 0.04 | ns | 0.44 and 0.049 |
| Percentage activated/memory and CD4+ T-cell count | ns | −0.90 and 0.04 | ns | −0.54 and 0.014 |
| CXCR4 in T CD4+(MFI) | ||||
| Total | 586 (189-805) | 572 (190-1231) | 500 (248-610) | 199 (47-639)***,† |
| NV | 912 (610-1010) | 927 (712-1425) | 897 (745-958) | 287 (112-314)***,† |
| CM | 294 (112-357) | 274 (97-412) | 257 (100-387) | 124 (58-220)*,† |
| EM | 151 (92-245) | 167 (102-258) | 102 (56-257) | 64 (42-112)**,† |
| TEMRA | 382 (212-478) | 427 (247-528) | 450 (312-524) | 77 (55-97)***,† |
| CXCR4 in T CD4+(%) | ||||
| Total | 61.5 (38.4-75.7) | 67 (37.2-89.3) | 60.4 (42.1-76.2) | 39.5 (12.4-65.7)**,† |
| NV | 80.2 (64.1-94-1) | 84.3 (68.2-97.4) | 77.5 (55.2-84.3) | 39.1 (12.6-48.4)***,† |
| CM | 53.2 (44.7-67.7) | 56.1 (45.3-71.3) | 49.7 (32.1-55.4) | 32.4 (22.8-48.6)*,† |
| EM | 40.7 (35.1-52.1) | 39.3 (42.1-64.8) | 42.4 (22.4-58.4) | 24.9 (11.3-28.7)**,† |
| TEMRA | 59.5 (32.4-64.4) | 66.2 (42.1-77.3) | 51.8 (33.7-64.7) | 26.4 (8.3-32.7)**,† |
| Correlation factor (r Spearman’s and P value) | ||||
| CXCR4 MFI and CD4+ T-cell count | ns | ns | ns | 0.45 and 0.039 |
| CXCR4 % and CD4+ T-cell count | ns | ns | ns | 0.46 and 0.035 |
Data are medians (ranges). Using the protein tyrosine phosphatase isoform CD45RA and the chemokine receptor CCR7, CD4+ T cells were subdivided into naive (NV), central memory (CM), effector memory (EM), and terminally differentiated effector memory (TEMRA) subpopulations. All P values are compared with healthy individuals.
ns, not significant.
P < .05.
P < .005.
P < .0005.
Significantly different compared with elderly subjects (as determined using the Mann-Whitney U test).
Absence of association between the rate of infections and the senescent state of T cells in patients with ICL
| Characteristics . | Asymptomatic (n = 8) . | Opportunistic infections (n = 12) . | P value . | CMV-negative (n = 12) . | CMV-positive (n = 8) . | P value . |
|---|---|---|---|---|---|---|
| CD4+ T-cell count, cells/mm3 | 162 (38-278) | 40.5 (22-219) | .05 | 100 (25-222) | 187 (22-278) | .5 |
| CD4+ T cells, % | 35.5 (3-62) | 19 (5-29) | .04 | 27 (3-62) | 23 (5-40) | .36 |
| CD27−CD28− in CD4+ T cells, % | 23.5 (3.3-30.7) | 27.5 (18.9-59.1) | .06 | 25.8 (3.3-56.4) | 23.2 (10.7-59.1) | .89 |
| KLRG1+CD57+ in CD4+ T cells, % | 7.2 (4.2-13.2) | 13 (4.5-46) | .09 | 11.3 (4.2-33.1) | 13.75 (5.5-46) | .32 |
| CD8+ T-cell count, cells/mm3 | 104 (46-691) | 102.5 (15-392) | .67 | 55 (15-691) | 132 (25-325) | .97 |
| CD8+ T cells, % | 18 (13-57) | 17.5 (9-77) | .65 | 15 (10-57) | 20 (9-77) | .3 |
| CD27−CD28− in CD8+ T cells, % | 26.3 (6.37-49.6) | 32.3 (19.8-56.4) | .20 | 32.1 (19.8-55.2) | 32.30 (6.37-56.4) | .57 |
| KLRG1+CD57+ in CD8+ T cells, % | 28 (4.4-35.6) | 22.9 (11.5-48.3) | .80 | 23.2 (4.4-48.3) | 32.25 (11.5-43.1) | .58 |
| Characteristics . | Asymptomatic (n = 8) . | Opportunistic infections (n = 12) . | P value . | CMV-negative (n = 12) . | CMV-positive (n = 8) . | P value . |
|---|---|---|---|---|---|---|
| CD4+ T-cell count, cells/mm3 | 162 (38-278) | 40.5 (22-219) | .05 | 100 (25-222) | 187 (22-278) | .5 |
| CD4+ T cells, % | 35.5 (3-62) | 19 (5-29) | .04 | 27 (3-62) | 23 (5-40) | .36 |
| CD27−CD28− in CD4+ T cells, % | 23.5 (3.3-30.7) | 27.5 (18.9-59.1) | .06 | 25.8 (3.3-56.4) | 23.2 (10.7-59.1) | .89 |
| KLRG1+CD57+ in CD4+ T cells, % | 7.2 (4.2-13.2) | 13 (4.5-46) | .09 | 11.3 (4.2-33.1) | 13.75 (5.5-46) | .32 |
| CD8+ T-cell count, cells/mm3 | 104 (46-691) | 102.5 (15-392) | .67 | 55 (15-691) | 132 (25-325) | .97 |
| CD8+ T cells, % | 18 (13-57) | 17.5 (9-77) | .65 | 15 (10-57) | 20 (9-77) | .3 |
| CD27−CD28− in CD8+ T cells, % | 26.3 (6.37-49.6) | 32.3 (19.8-56.4) | .20 | 32.1 (19.8-55.2) | 32.30 (6.37-56.4) | .57 |
| KLRG1+CD57+ in CD8+ T cells, % | 28 (4.4-35.6) | 22.9 (11.5-48.3) | .80 | 23.2 (4.4-48.3) | 32.25 (11.5-43.1) | .58 |
Data are medians (ranges). Patients were distributed according to the opportunistic infection or serological (negative, IgM− IgG−; or positive: IgM− IgG+) CMV status. P values were determined using the Mann-Whitney U test.
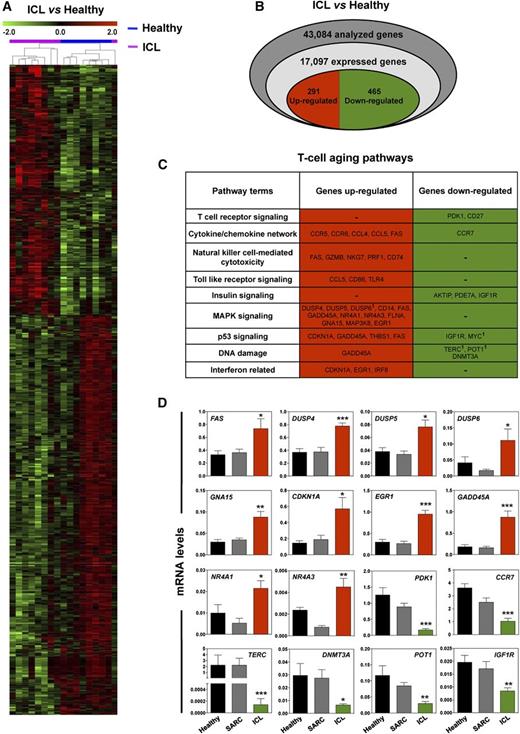
Gene expression signatures of ICL CD4+ T cells
To gain some mechanistic insight into the functional pathways involved in ICL pathophysiology, we used Affymetrix Exon Arrays to compare the CD4+ T-cell gene expression profiles of representative patients with ICL (n = 9) according to age, sex, and opportunistic infection status with those obtained from subjects in the healthy (n = 8) and SARC (n = 8) control groups. Differential gene expression analyses were performed for patients with ICL vs healthy subjects (Figure 1A; supplemental Table 4) and for patients with ICL vs patients with SARC (supplemental Table 5). Comparisons between healthy individuals and patients with SARC revealed only a few differentially regulated genes in the CD4+ T cells from patients with SARC (supplemental Table 6). In contrast, we found a gene expression signature for the ICL CD4+ T cells compared with the healthy and SARC CD4+ T cells. We identified 291 upregulated and 465 downregulated genes in ICL vs healthy T cells with fold-changes of 1.5 or higher and uncorrected P values ≤ 0.05 (Figure 1B; supplemental Table 4).
Aging gene-expression profiles in CD4+ T cells from patients with ICL. (A) Regulated genes across the samples were hierarchically clustered according to their mean-normalized intensity across all samples. Blue and pink samples correspond to CD4+ T cells from healthy subjects (n = 8) and patients with ICL (n = 9), respectively. (B) Venn diagram of analyzed, expressed, and regulated genes in CD4+ T cells for patients with ICL vs healthy subjects. Upregulated and downregulated genes are indicated by red and green colors, respectively. (C) Results extracted from the Gene Ontology analysis, KEGG pathway, and list of genes regulated in healthy T cells during aging28 were combined to delineate a specific T-cell aging pathway in ICL. 1Genes that are significantly regulated in another hierarchical clustering. (D) Ten representative genes of the upregulated group and 6 of the downregulated group were monitored by quantitative PCR in healthy (n = 12), SARC (n = 8), and ICL (n = 12) CD4+ T cells. Each individual sample was run in triplicate. Results are expressed as mRNA levels after normalization to GAPDH mRNA levels. *P < .05, **P < .005, and ***P < .0005 compared with healthy CD4+ T cells (as determined using the Mann-Whitney U test).
Aging gene-expression profiles in CD4+ T cells from patients with ICL. (A) Regulated genes across the samples were hierarchically clustered according to their mean-normalized intensity across all samples. Blue and pink samples correspond to CD4+ T cells from healthy subjects (n = 8) and patients with ICL (n = 9), respectively. (B) Venn diagram of analyzed, expressed, and regulated genes in CD4+ T cells for patients with ICL vs healthy subjects. Upregulated and downregulated genes are indicated by red and green colors, respectively. (C) Results extracted from the Gene Ontology analysis, KEGG pathway, and list of genes regulated in healthy T cells during aging28 were combined to delineate a specific T-cell aging pathway in ICL. 1Genes that are significantly regulated in another hierarchical clustering. (D) Ten representative genes of the upregulated group and 6 of the downregulated group were monitored by quantitative PCR in healthy (n = 12), SARC (n = 8), and ICL (n = 12) CD4+ T cells. Each individual sample was run in triplicate. Results are expressed as mRNA levels after normalization to GAPDH mRNA levels. *P < .05, **P < .005, and ***P < .0005 compared with healthy CD4+ T cells (as determined using the Mann-Whitney U test).
To identify the canonical pathways differentially modulated in ICL, the ICL signature transcripts were subjected to Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analyses. The T-cell genes differentially expressed in ICL were enriched in functional groups associated with telomere shortening, defective T-cell signaling, altered immune responses, impaired DNA repair, and p53 signaling (Figure 1C), which are related to cellular senescence. Subsequently, the ICL signature was compared with a list of genes known to change expression levels with increasing age in T cells.28 Some of the most highly upregulated genes in ICL CD4+ T cells included the DUSP family members DUSP4, DUSP5, and DUSP6, which are MAPK phosphatases that negatively regulate TCR signaling and the immune response.29-31 We also found that the cell cycle inhibitors CDKN1A, GADD45A, and FAS, all of which are upregulated with increasing age in T cells,28 were also upregulated in ICL. A striking observation was the marked downregulation in ICL CD4+ T cells of TERC, POT1, and DNMT3A, which are involved in the senescence process. We confirmed the microarray data for selected transcripts by real-time PCR (Figure 1D). These results highlight an ICL-related transcriptional signature suggestive of a diminished TCR response and enhanced aging.
Decreased sensitivity to TCR stimulation in ICL T cells
According to our transcriptomic analyses, we investigated the strength of the TCR signaling in ICL CD4+ T cells. These analyses were extended to include T cells from 6 nonagenarian subjects that, similar to ICL T cells, displayed an inflation of the TEMRA phenotype but exhibited normal to high levels of membrane CXCR4 expression and function (Tables 1 and 2; supplemental Tables 2 and 3).32-34 First, we assessed TCR responsiveness by stimulating CFSE-loaded PBMCs from healthy, elderly, SARC, and ICL subjects on anti-CD3/anti-CD28 Ab-coated plates for 5 days (Figure 2A-B). As expected,31,35 CD4+ T cells from elderly and SARC subjects exhibited impaired proliferation after TCR engagement. TCR-mediated proliferation was also significantly lower in ICL CD4+ T cells than in healthy cells. Responsiveness to TCR stimulation was similarly affected in ICL CD8+ T cells (supplemental Figure 3A-B). To probe early signaling events after TCR cross-linking, we assessed the phosphorylation of extracellular signal-regulated kinase 1 (ERK-1) and ERK-2 MAPKs by PhosphoFlow. TCR-induced ERK activation was markedly lower in CD4+ and CD8+ T cells from elderly, SARC, and ICL individuals than in healthy T cells (Figure 2C-D; supplemental Figure 3C-D). These findings indicate that the TCR response is diminished in ICL T cells. This low level of TCR activation is comparable to that found in elderly subjects, whose T cells are known to be senescent, and in patients with SARC, whose T cells are known to be anergic.31,35
Low responsiveness of ICL CD4+ T cells to TCR stimulation. (A and B) PBMCs from healthy (n = 28), elderly (n = 6), SARC (n = 8), and ICL (n = 20) individuals were CFSE-labeled and stimulated on anti-CD3/anti-CD28 Ab-coated plates or left untreated (basal) for 5 days. Fluorescence intensity loss resulting from division cycles was determined by flow cytometry. Representative plots of the mean fluorescence intensity (MFI) of CFSE in CD4+-gated T cells are shown (A). Results (mean ± standard deviation [SD] of 10 independent experiments) are expressed as percentages of proliferating CFSElow (>1 cell division) CD4+ T cells (B). (C and D) PBMCs were stimulated for 5 min by CD3 cross-linking or left untreated and immediately analyzed by PhosphoFlow for intracellular phospho-ERK-1/2 (pERK) content. Representative plots of the MFI of pERK in CD4+-gated T cells are shown. Background fluorescence (shaded area) was assessed using an isotype control Ab (C). The bar graphs summarize the results (expressed as mean ± SD) from all analyzed subjects (D). Kruskal-Wallis H test and associated P values are indicated. *P < 0.05 and ***P < 0.0005 compared with healthy CD4+ T cells (as determined using the Mann-Whitney U test).
Low responsiveness of ICL CD4+ T cells to TCR stimulation. (A and B) PBMCs from healthy (n = 28), elderly (n = 6), SARC (n = 8), and ICL (n = 20) individuals were CFSE-labeled and stimulated on anti-CD3/anti-CD28 Ab-coated plates or left untreated (basal) for 5 days. Fluorescence intensity loss resulting from division cycles was determined by flow cytometry. Representative plots of the mean fluorescence intensity (MFI) of CFSE in CD4+-gated T cells are shown (A). Results (mean ± standard deviation [SD] of 10 independent experiments) are expressed as percentages of proliferating CFSElow (>1 cell division) CD4+ T cells (B). (C and D) PBMCs were stimulated for 5 min by CD3 cross-linking or left untreated and immediately analyzed by PhosphoFlow for intracellular phospho-ERK-1/2 (pERK) content. Representative plots of the MFI of pERK in CD4+-gated T cells are shown. Background fluorescence (shaded area) was assessed using an isotype control Ab (C). The bar graphs summarize the results (expressed as mean ± SD) from all analyzed subjects (D). Kruskal-Wallis H test and associated P values are indicated. *P < 0.05 and ***P < 0.0005 compared with healthy CD4+ T cells (as determined using the Mann-Whitney U test).
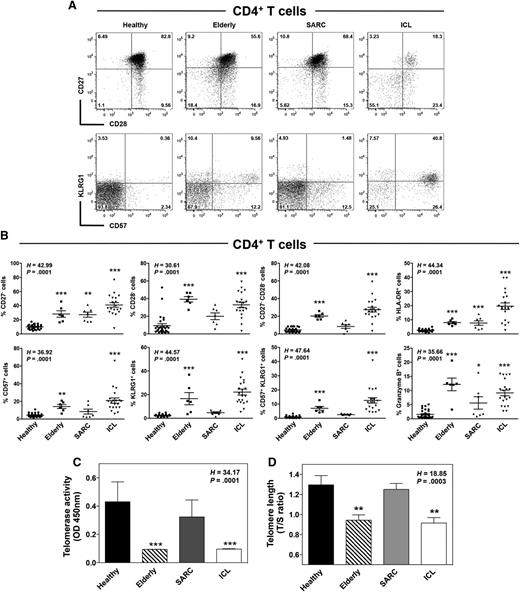
Accelerated T-cell senescence in ICL
Reduced TCR signaling intensity and impaired T-cell expansion in response to TCR stimulation are features of T-cell aging or anergy.36 We thus performed phenotyping analyses for 4 key markers of T-cell senescence: the costimulatory molecules CD27 and CD28 and the natural killer-cell markers CD57 and KLRG-1. As reported,34 CD4+ T cells from elderly subjects exhibited lower CD27 and CD28 expression levels and higher CD57 and KLRG-1 expressions than those of healthy subjects (Figure 3A-B). This was associated with higher expression of the cytotoxic molecule granzyme B. Although expression levels of these markers were scattered over a large range among patients with ICL, the percentages of CD27−, CD28−, CD57+, KLRG-1+, and granzyme B+ CD4+ T cells were significantly higher in this group than in healthy donors. Strikingly, the larger double populations of KLRG1+CD57+ and CD27−CD28− T cells (with an effector memory or TEMRA phenotype) in elderly subjects compared with those in healthy individuals were even more accentuated in patients with ICL. These cells are considered terminally differentiated effector cells and may arise from replicative senescence triggered by repeated antigenic stimulation.34,36 Consistent with chronic immune activation in ICL was the induction of the activation marker HLA-DR in CD4+ T cells (Figure 3B). We found similar expression patterns for these markers in the CD8+ T-cell compartment of these subjects (supplemental Figure 4). Combined with the CD45RA marker, the CD31 status is used to distinguish thymus-derived naive T cells (CD45RA+CD31+) from peripherally expanded naive T cells with homeostatic proliferative history (CD45RA+CD31−).37 We noticed no change in the frequency of CD45RA+CD31+CD4+ T cells in patients with ICL, whereas this population was decreased in the elderly group (supplemental Figure 5A-B). Interestingly, the proportion of CD45RA+CD31−CD4+ T cells was markedly decreased in the ICL group.
CD4+ T cells exhibit a senescent profile in ICL. (A and B) Flow-cytometric analyses of CD27, CD28, CD57, and KLRG1 expression in CD3+CD4+-gated PBMCs from healthy, elderly, SARC, and ICL subjects. Quadrants were set on controls stained with the corresponding isotype control Ab. Representative dot plots showing coexpression of CD27 and CD28 or CD57 and KLRG1 are shown (A). Comparison of the frequencies of CD27−, CD28−, HLADR−, CD57−, KLRG1−, and granzyme B-expressing CD4+ T cells in healthy (n = 28), elderly (n = 6), SARC (n = 8), and ICL (n = 20) individuals. Lines indicate the mean ± SD values, and each symbol represents the value from an individual (B). (C and D) Telomerase activity (C) and telomere length (D) were measured in PBMCs from the aforementioned groups by Telomere Repeat Amplification Protocol, followed by enzyme-linked immunosorbent assay detection and quantitative PCR, respectively. Results (mean ± SD) are from 3 independent experiments and show the optical density values (C) or are expressed as the ratio of telomere repeat copy number (T) to albumin copy number (a single-copy gene, S) within the same DNA sample in each group (D). Kruskal-Wallis H test values and associated P values are indicated. *P < 0.05, **P < 0.005, and ***P < 0.0005 compared with healthy leukocytes (as determined using the Mann-Whitney U test).
CD4+ T cells exhibit a senescent profile in ICL. (A and B) Flow-cytometric analyses of CD27, CD28, CD57, and KLRG1 expression in CD3+CD4+-gated PBMCs from healthy, elderly, SARC, and ICL subjects. Quadrants were set on controls stained with the corresponding isotype control Ab. Representative dot plots showing coexpression of CD27 and CD28 or CD57 and KLRG1 are shown (A). Comparison of the frequencies of CD27−, CD28−, HLADR−, CD57−, KLRG1−, and granzyme B-expressing CD4+ T cells in healthy (n = 28), elderly (n = 6), SARC (n = 8), and ICL (n = 20) individuals. Lines indicate the mean ± SD values, and each symbol represents the value from an individual (B). (C and D) Telomerase activity (C) and telomere length (D) were measured in PBMCs from the aforementioned groups by Telomere Repeat Amplification Protocol, followed by enzyme-linked immunosorbent assay detection and quantitative PCR, respectively. Results (mean ± SD) are from 3 independent experiments and show the optical density values (C) or are expressed as the ratio of telomere repeat copy number (T) to albumin copy number (a single-copy gene, S) within the same DNA sample in each group (D). Kruskal-Wallis H test values and associated P values are indicated. *P < 0.05, **P < 0.005, and ***P < 0.0005 compared with healthy leukocytes (as determined using the Mann-Whitney U test).
To characterize the senescent profile of ICL cells, we analyzed telomerase activity and telomere length, which are modulated in part by CD27 or CD28 signaling and are major markers of cellular aging, including that of lymphocytes.38-40 Telomeres consist of hexanucleotide repeats at the ends of linear chromosomes, together with several associated proteins, some of which, such as POT1, are downregulated in ICL CD4+ T cells (Figure 1). Telomerase is a ribonucleoprotein DNA polymerase consisting of 2 components: TERT, a catalytic reverse transcriptase, and TERC, an RNA template that encodes the telomeric DNA repeats. We found both of these to be downregulated in ICL (Figure 1). The level of telomerase activity was lower in ICL and elderly PBMCs than in healthy or SARC PBMCs (Figure 3C), and this was associated with shortened telomere length (Figure 3D). To distinguish these observations from exhaustion, we analyzed in ICL T cells the expression levels of the inhibitory receptors PD-1 and CTLA-4.41 We observed no increase in any of those markers on CD4+ T cells from patients with ICL compared with those markers on healthy cells (supplemental Figure 5A-B). This was associated with no change in the expression of PRDM1 that encodes B lymphocyte-induced maturation protein-1, a transcriptional repressor highly expressed in exhausted T cells and responsible for the increased expression of coinhibitory receptors (supplemental Figure 5C).42,43 In addition, ICL T cells were capable of producing significant amounts of interferon-γ after TCR stimulation (supplemental Figure 5D). Combined with the fact that ICL T cells displayed increased expression of senescence-related genes such as CDKN1A and GADD45A (Figure 1), these results suggest that circulating memory T cells from patients with ICL exhibit an untimely senescent, rather than exhausted, profile similar to that of T cells from healthy elderly individuals.
No effect of opportunistic or chronic infections on T-cell senescence in ICL
There was no significant correlation between the age or T-cell count of patients with ICL and the expression levels of senescent markers (supplemental Table 7). We sought to determine whether T-cell changes in ICL could result from opportunistic or chronic infections. When asymptomatic patients (n = 8) were compared with those who had experienced opportunistic infections (n = 12), no significant difference was obtained for the senescence markers (Table 3). Similarly, the cytomegalovirus (CMV) serological status did not affect the expression levels of senescent markers (Tables 1 and 3). In particular, CD4+ T cells from the youngest patient (24 years old) harbored one of the highest levels of senescence markers (eg, 23.2% CD57+KLRG1+CD4+ T cells and 32.4% CD27−CD28−CD4+ T cells). This patient was CMV IgM- and IgG-negative and had no history of opportunistic infection. In contrast, the oldest patient (76 years old), who belongs to the group that displayed less marked T-cell senescence (eg, 6.2% CD57+KLRG1+CD4+ T cells and 22.5% CD27−CD28−CD4+ T cells), was CMV IgG-positive and presented clinical signs of Pneumocystis jirovecii pneumonia. These findings suggest that accelerated aging in ICL is likely not related to the presence of subsequent infections.
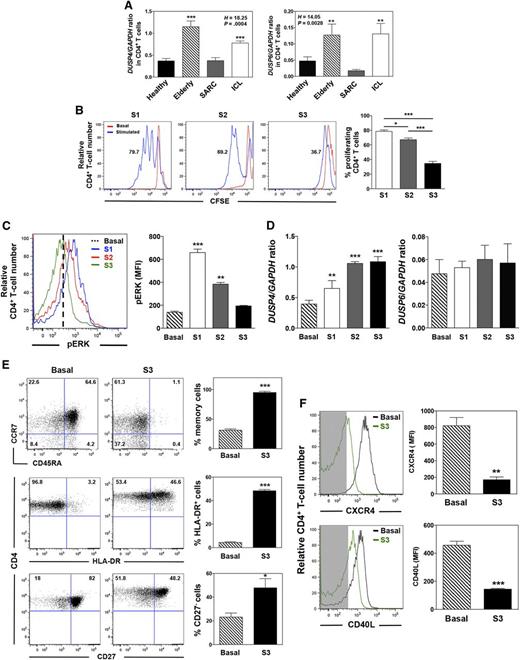
Repeated TCR stimulation leads to DUSP4 overexpression
One feedback mechanism that regulates the nuclear activities of the MAPK pathway and dampens TCR signaling involves the DUSP family of phosphatases.44 Increased expression of DUSP6 and DUSP4 with age has been reported in naive and memory CD4+ T cells, respectively.29,31 To elucidate the mechanism underlying the decrease in TCR-induced ERK activation in expanded-memory T cells from patients with ICL, we investigated the DUSP4 status. As determined by real-time PCR, CD4+ T cells from elderly and patients with ICL exhibited higher DUSP4 mRNA levels than those of healthy individuals and patients with SARC (Figure 4A). This was also detected for DUSP6 in accordance with transcriptomic analyses (Figure 1D). To investigate the putative chronic stimulation that has been suggested to occur in ICL,19 we determined DUSP4 behavior in response to 3 repeated rounds of stimulation with anti-CD3/anti-CD28 Abs that lead to successive induction of TCR-mediated responses. Ultimately, chronically activated T cells reach a senescent profile and do not respond to further stimulation.45 In healthy CD4+ T cells, repeated TCR engagement led to impaired induction of the proliferative response and to lower ERK phosphorylation (Figure 4B-C). This was associated with a gradual increase in DUSP4, but not DUSP6, expression (Figure 4D). Two rounds of TCR stimulation were sufficient to give rise to DUSP4 mRNA levels similar to those detected in nonmanipulated elderly and ICL cells (Figure 4A). DUSP4-overexpressing T cells displayed increased expression of HLA-DR in contrast to the strong decline in surface expression of CD45RA, CXCR4, and the costimulatory molecules CD27 and CD40L (Figure 4E-F). This expression pattern is consistent with a terminally differentiated phenotype.29,34 These findings suggest that memory-activated CD4+ T cells respond poorly to successive TCR stimuli and that the consequent increase in DUSP4 expression constitutes negative regulatory feedback.
Repeated TCR stimulation leads to defective signaling and DUSP4 overexpression in healthy CD4+ T cells. (A) Steady-state levels of DUSP4 and DUSP6 transcripts in sorted CD4+ T cells from healthy (n = 28), elderly (n = 6), SARC (n = 8), and ICL (n = 20) subjects were assessed by quantitative PCR. Each individual sample was run in triplicate. Results are expressed as DUSP4 or DUSP6/GAPDH ratio. (B) CFSE-loaded CD4+ T cells from 9 independent healthy donors were either left untreated (basal) or successively stimulated once, twice, or 3 times (S1-S3) on anti-CD3/anti-CD28 Ab-coated plates for 5 days. Each round of stimulation was separated by a 2-day culture in complete RPMI medium without any stimulation. Representative plots of the MFI of CFSE in CD4+ T cells (left). Results (mean ± SD of 3 independent experiments) expressed as fractions of proliferating CFSElow CD4+ T cells (right). Cells were gated on forward and side scatter to eliminate debris and on forward scatter-width/area and side scatter-width/area to gate only single and viable cells. Samples contained between 46.5% and 87.3% viable cells. (C) pERK content was determined in CD4+ T cells after restimulation by CD3 cross-linking for 5 minutes. Representative plots of the MFI of pERK in CD4+ T cells (left). Mean pERK content in unstimulated and activated CD4+ T cells ± SD (right). (D) DUSP4 and DUSP6 mRNA levels (mean ± SD, n = 9) were evaluated by quantitative PCR in healthy CD4+ T cells left untreated or repeatedly stimulated with anti-CD3/anti-CD28 Abs. (E and F) Flow-cytometric analyses of CD45RA, HLA-DR, CD27, CD40L, CCR7, and CXCR4 expression on healthy CD4+ T cells left untreated or after 3 rounds of stimulation. Representative dot plots or histograms (left). Background fluorescence is shown as shaded areas in F. Frequencies (mean ± SD, n = 9) of naive (CD45RA+CCR7+) vs memory (CD45RA−CCR7−/+), HLA-DR-expressing, and CD27-expressing CD4+ T cells (E) and levels of CXCR4 and CD40L expression (F; right). Kruskal-Wallis H test values and associated P values are indicated. *P < 0.05, **P < 0.005, and ***P < 0.0005 compared with healthy (A), untreated (C-F), or stimulated-S1 (B) CD4+ T cells (as determined using the Mann-Whitney U test).
Repeated TCR stimulation leads to defective signaling and DUSP4 overexpression in healthy CD4+ T cells. (A) Steady-state levels of DUSP4 and DUSP6 transcripts in sorted CD4+ T cells from healthy (n = 28), elderly (n = 6), SARC (n = 8), and ICL (n = 20) subjects were assessed by quantitative PCR. Each individual sample was run in triplicate. Results are expressed as DUSP4 or DUSP6/GAPDH ratio. (B) CFSE-loaded CD4+ T cells from 9 independent healthy donors were either left untreated (basal) or successively stimulated once, twice, or 3 times (S1-S3) on anti-CD3/anti-CD28 Ab-coated plates for 5 days. Each round of stimulation was separated by a 2-day culture in complete RPMI medium without any stimulation. Representative plots of the MFI of CFSE in CD4+ T cells (left). Results (mean ± SD of 3 independent experiments) expressed as fractions of proliferating CFSElow CD4+ T cells (right). Cells were gated on forward and side scatter to eliminate debris and on forward scatter-width/area and side scatter-width/area to gate only single and viable cells. Samples contained between 46.5% and 87.3% viable cells. (C) pERK content was determined in CD4+ T cells after restimulation by CD3 cross-linking for 5 minutes. Representative plots of the MFI of pERK in CD4+ T cells (left). Mean pERK content in unstimulated and activated CD4+ T cells ± SD (right). (D) DUSP4 and DUSP6 mRNA levels (mean ± SD, n = 9) were evaluated by quantitative PCR in healthy CD4+ T cells left untreated or repeatedly stimulated with anti-CD3/anti-CD28 Abs. (E and F) Flow-cytometric analyses of CD45RA, HLA-DR, CD27, CD40L, CCR7, and CXCR4 expression on healthy CD4+ T cells left untreated or after 3 rounds of stimulation. Representative dot plots or histograms (left). Background fluorescence is shown as shaded areas in F. Frequencies (mean ± SD, n = 9) of naive (CD45RA+CCR7+) vs memory (CD45RA−CCR7−/+), HLA-DR-expressing, and CD27-expressing CD4+ T cells (E) and levels of CXCR4 and CD40L expression (F; right). Kruskal-Wallis H test values and associated P values are indicated. *P < 0.05, **P < 0.005, and ***P < 0.0005 compared with healthy (A), untreated (C-F), or stimulated-S1 (B) CD4+ T cells (as determined using the Mann-Whitney U test).
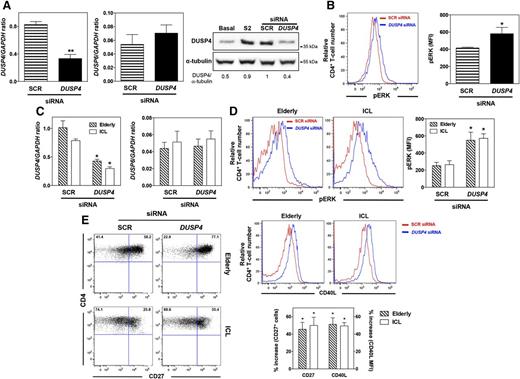
DUSP4 silencing improves T-cell activity in ICL
To determine whether DUSP4 negatively regulates TCR signaling, the activation-induced increase in the level of DUSP4 mRNAs was silenced in healthy CD4+ T cells after 2 rounds of stimulation. Quantitative PCR and immunoblot analyses revealed that DUSP4 knockdown reduced expression of DUSP4 by more than 60% compared with the scrambled control (SCR) siRNA (Figure 5A). This was apparently specific, as DUSP4 siRNA did not affect DUSP6 expression and permitted DUSP4 expression to return to the basal level. Reduction of DUSP4 mRNA levels improved TCR-induced ERK phosphorylation in activated CD4+ T cells compared with that in cells transfected with a SCR siRNA (Figure 5B). We next reasoned that DUSP4 silencing could at least partially ameliorate CD4+ T-cell activation in patients with ICL and elderly subjects.29 Normalization of DUSP4 expression in elderly and ICL CD4+ T cells restored TCR-induced ERK phosphorylation (Figures 4A and 5C-D). On day 3 after stimulation, expression levels of CD27 and CD40L in CD4+ T cells from elderly individuals and patients with ICL increased after DUSP4 knockdown (Figure 5E). These results indicate that intrinsic CD4+ T-cell defects in ICL partially depend on high expression of DUSP4 and that its silencing was able to reverse T-cell unresponsiveness.
DUSP4 inhibition ameliorates TCR signaling in senescent CD4+ T cells. (A and B) After 2 rounds of stimulation with anti-CD3/anti-CD28 Abs, activated CD4+ T cells from 3 independent healthy individuals were nucleoporated with 1.5 μg SCR or DUSP4 siRNAs. Twelve hours after transfection, T cells were stimulated on plates coated with anti-CD3/anti-CD28 Abs for 2 days. DUSP4 and DUSP6 transcript or protein levels were then evaluated by quantitative PCR (left and middle) or immunoblot (right). The ratios of DUSP4 over α-tubulin proteins are indicated below the gels. pERK content was determined by PhosphoFlow in siRNA-transfected T cells restimulated by CD3 cross-linking (B). Representative plots of the MFI of pERK in transfected CD4+ T cells are shown (left). Results show the mean pERK content in transfected CD4+ T cells ± SD (right). (C-E) CD4+ T cells from 3 independent elderly or ICL subjects were nucleoporated with 1.5 μg SCR or DUSP4 siRNAs and then stimulated for 2 days, as described earlier. siRNA-transfected cells were recovered and tested for DUSP4 and DUSP6 expression (C) and for their pERK content after CD3 cross-linking (D). On day 3 after stimulation, the frequencies of CD27-expressing cells CD4+ T cells and levels of CD40L expression were determined by flow cytometry and shown as the percentage increase after DUSP4 silencing (E). Results represent the mean ± SD or are representative of 3 independent experiments. *P < 0.05 and **P < 0.005 compared with SCR siRNA-transfected CD4+ T cells (as determined using the Mann-Whitney U test).
DUSP4 inhibition ameliorates TCR signaling in senescent CD4+ T cells. (A and B) After 2 rounds of stimulation with anti-CD3/anti-CD28 Abs, activated CD4+ T cells from 3 independent healthy individuals were nucleoporated with 1.5 μg SCR or DUSP4 siRNAs. Twelve hours after transfection, T cells were stimulated on plates coated with anti-CD3/anti-CD28 Abs for 2 days. DUSP4 and DUSP6 transcript or protein levels were then evaluated by quantitative PCR (left and middle) or immunoblot (right). The ratios of DUSP4 over α-tubulin proteins are indicated below the gels. pERK content was determined by PhosphoFlow in siRNA-transfected T cells restimulated by CD3 cross-linking (B). Representative plots of the MFI of pERK in transfected CD4+ T cells are shown (left). Results show the mean pERK content in transfected CD4+ T cells ± SD (right). (C-E) CD4+ T cells from 3 independent elderly or ICL subjects were nucleoporated with 1.5 μg SCR or DUSP4 siRNAs and then stimulated for 2 days, as described earlier. siRNA-transfected cells were recovered and tested for DUSP4 and DUSP6 expression (C) and for their pERK content after CD3 cross-linking (D). On day 3 after stimulation, the frequencies of CD27-expressing cells CD4+ T cells and levels of CD40L expression were determined by flow cytometry and shown as the percentage increase after DUSP4 silencing (E). Results represent the mean ± SD or are representative of 3 independent experiments. *P < 0.05 and **P < 0.005 compared with SCR siRNA-transfected CD4+ T cells (as determined using the Mann-Whitney U test).
Discussion
In this study, we found that the characteristics of T cells from 20 adults with ICL closely mimic T-cell alterations that normally occur during aging. First, ICL T cells displayed a decrease in the naive subset and an expansion of the TEMRA subpopulation in the blood. This phenotype is observed in elderly individuals, for whom it could reflect the cumulative exposure of T cells to pathogens throughout life.36,46 However, the decreased frequency of CD45RA+CD31− in ICL CD4+ T cells was mirrored by an increased fraction of CD45RA−CD31− cells, presumably of memory/activated phenotype. Thus, the CD4 lymphocytopenia that affects predominantly the naive compartment could reflect impairment in homeostatic proliferation of naive T cells47 and peripheral accelerated consumption associated with conversion of naive into effector/memory cells. Second, our transcriptomic study is the first to provide a comprehensive description of the gene expression profile in ICL CD4+ T cells. We observed in ICL the same alterations found in the aging-related gene networks and signaling pathways of elderly individuals.28 Third, similar to elderly T cells,34,46 ICL T cells displayed defective TCR responses, low levels of CD27 and CD28, high expressions of natural killer cell markers, telomere shortening, and down-modulation of regulatory genes such as TERC and POT1. This profile was further implemented by the preserved ability of ICL T cells to secrete interferon-γ and by the absence of upregulation of exhaustion markers.41,48,49 Thus, patients with ICL may encounter an accelerated T-cell senescence, rather than exhaustion.
In older adults, the loss of CD28 is clearly associated with immune senescence, and therefore, with the decline in immune responsiveness. CD28 loss occurs after persistent antigenic stimulation and after each cycle of proliferation, which in both cases leads to an accumulation of TEMRA T cells.50 Telomere shortening throughout the life span is a result of the repeated activation of T cells by antigens, and accelerated telomere erosion is found in diseases associated with chronic immune activation, such as rheumatoid arthritis and AIDS.38 Interestingly, ICL resembles HIV from a clinical view, but also from the pathophysiological view, with accelerated immune aging.51,52 Several studies have demonstrated that TEMRA T cells cannot halt telomere shortening because they lack the mechanisms that compensate for telomere erosion. One such mechanism is POT1-mediated maintenance,36 which is also altered in ICL T cells. Therefore, T-cell overstimulation may be one cause of the accelerated aging phenotype found in ICL T cells.
We detected high DUSP4 expression in ICL CD4+ T cells. This is physiologically relevant and consistent with the terminally differentiated, activated, and senescent profile associated with T cells expressing an anergic TCR.29 Similar to DUSP6,53 DUSP4 dephosphorylates ERK and c-Jun N-terminal kinases,54,55 but also signal transducer and activator of transcription 5, and thereby regulates IL-2 signaling.56 In vitro, we have shown that DUSP4, but not DUSP6, transcription is upregulated after successive TCR engagement in healthy CD4+ T cells, suggesting it constitutes a negative feedback that prevents excessive T-cell activation by halting MAPK phosphorylation. ICL T cells are similar to elderly T cells in this respect.29 In this context, DUSP4 is a 2-edged sword that may prevent excessive T-cell activation, but in contrast, also inhibits TCR responses. This limits T-cell responses to antigens, likely impairs the ability of T cells to provide help to B cells, and may account for the poor responses to vaccines seen in older adults and patients with ICL.57
DUSP4 overexpression in ICL CD4+ T cells is likely secondary to chronic TCR stimulation and reflects either the activation strength of T cells or their cumulative number of cell cycles or both. This model agrees well with the anergic TCR, low telomerase activity, and short telomeres observed in ICL cells. Primitive overexpression of DUSP4 in ICL would likely have prevented the T-cell proliferation, differentiation, and telomere shortening. Elevated DUSP4 expression in memory CD4+ T cells of older adults is caused by augmented transcription, rather than by epigenetic mechanisms. Previous works have reported that DUSP4 expression after TCR activation depends on both the expression and activity of the transcription factor EGR1.29,58 EGR1 expression is higher in elderly T cells than in those of young adults. In line with this, our transcriptomic and real-time PCR approaches revealed that EGR1 is upregulated in ICL CD4+ T cells, which likely accounts for the high levels of DUSP4 mRNA found in these cells.
Another important question concerns the mechanisms leading to chronic T-cell stimulation in ICL. Neither opportunistic infections nor a chronic viral infection such as CMV were found to be associated with T-cell changes in ICL. Although the effect of unappreciated infections cannot be excluded, we hypothesize that accelerated aging in ICL does reflect a cumulative history of T-cell stimulation induced by extrinsic or intrinsic factors. In line with a study describing a potential association between microbial dysbiosis and perturbed CD4+ T-cell homeostasis in ICL,19 microbial antigens may play a role in the aging phenotype. Alternatively, TCR dysfunction could be intrinsically a result of a dysregulated protein kinase or phosphatase downstream to the TCR. Examples of such dysregulations, including defective Lck, have been reported.8,20,59-61 Our results implicate another intracellular target (DUSP4) in these impaired TCR responses. DUSP4 silencing improved ICL TCR signaling in vitro and increased CD27 and CD40L expressions, which is likely to be consistent with the ability of DUSP4 knockdown to restore T-cell-dependent B-cell responses.29 In light of recent works pointing to mutations in RAG1, MAGT1, ITK, Unc119, and JAK3 in pediatric or young adult cases,3,4,8-10 it would be interesting to determine whether lymphocytopenia could be uncoupled, or not, from T-cell senescence in patients carrying a gene mutation.
Although T cells from patients with ICL and elderly subjects share several features, their CXCR4 expression levels were strikingly different, being 10-fold lower in ICL than in older adult T cells. This might reflect either CXCR4 internalization after strong TCR activation62 or a lack of CXCR4 recycling to the plasma membrane.12 One interpretation is that CXCR4 is re-expressed in elderly T cells because activation is less frequent or intense than in ICL T cells. This is supported by our in vitro data indicating that chronic stimulation of healthy T cells is associated with impaired membrane expression of CXCR4. The weak expression of CXCR4 detected on T cells from most of the patients, who exhibit heterogeneous clinical manifestations, suggests that loss of CXCR4 expression and function constitutes a common biologic trait of ICL.
Our study highlights that premature T-cell senescence occurs in ICL, likely as a result of chronic T-cell activation. This in part relies on the elevated expression of DUSP4, the modulation of which may constitute a novel therapeutic approach in ICL to improve the activity of the expanded-memory CD4+ T-cell pool.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr H. Gary (Institut Paris-Saclay d'Innovation Thérapeutique, Immunomonitoring Platform, Clamart, France) and Dr D. Gentien (Platform of Molecular Biology Facilities, Institut Curie, Paris, France) for their excellent technical assistance. We are grateful to Dr F. Bachelerie and Dr M. Espéli (INSERM UMR_S996, Clamart, France) for critical reading of the manuscript and Dr J. Harriague and Dr K. Liittschwager (4Clinics, Paris, France) for editing the manuscript.
The study was supported by Agence Nationale de la Recherche (ANR) grant 2010-JCJC-110401 (to K.B.), and by a grant from the Programme Hospitalier de Recherche Clinique, French Ministry of Health (2009-01-55; Lympho-4). A.B., L.K., A.D., V.M., A. Dalloul, and K.B. are members of the Laboratoire d'Excellence en Recherche sur le Médicament et l'Innovation Thérapeutique, supported by ANR grant ANR-10-LABX-33 under the program “Investissements d’Avenir” ANR-11-IDEX-0003-01. A.B., L.K., and A.D. were fellowship recipients from the French Ministry and from the Fondation pour la Recherche Médicale (FDT20130928127), the Laboratoire d'Excellence en Recherche sur le Médicament et l'Innovation Thérapeutique, and the Assistance Publique–Hôpitaux de Paris, respectively.
Authorship
Contribution: A.B. conceived, designed, and performed most of the experiments and contributed to manuscript writing; A.R. provided blood samples from elderly subjects and patients with ICL and reviewed the manuscript; L.K. performed some of the experiments and reviewed the manuscript; A. Desnoyer performed some of the experiments and reviewed the manuscript; P.d.l.G. analyzed microarray data and contributed to manuscript writing; V.M. provided blood samples from patients with SARC and reviewed the manuscript; O.L. provided blood samples from patients with ICL and reviewed the manuscript; A. Dalloul contributed to data analyses and manuscript writing. L.M. provided blood samples from older adult subjects and patients with ICL and reviewed the manuscript; and K.B. conceived and designed the study, found funding for the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl Balabanian, INSERM UMR_S996, Université Paris-Sud, LERMIT, 32 rue des Carnets, 92140 Clamart, France; e-mail: karl.balabanian@u-psud.fr.
References
Author notes
A.R. and L.K. contributed equally to this study.

![Figure 2. Low responsiveness of ICL CD4+ T cells to TCR stimulation. (A and B) PBMCs from healthy (n = 28), elderly (n = 6), SARC (n = 8), and ICL (n = 20) individuals were CFSE-labeled and stimulated on anti-CD3/anti-CD28 Ab-coated plates or left untreated (basal) for 5 days. Fluorescence intensity loss resulting from division cycles was determined by flow cytometry. Representative plots of the mean fluorescence intensity (MFI) of CFSE in CD4+-gated T cells are shown (A). Results (mean ± standard deviation [SD] of 10 independent experiments) are expressed as percentages of proliferating CFSElow (>1 cell division) CD4+ T cells (B). (C and D) PBMCs were stimulated for 5 min by CD3 cross-linking or left untreated and immediately analyzed by PhosphoFlow for intracellular phospho-ERK-1/2 (pERK) content. Representative plots of the MFI of pERK in CD4+-gated T cells are shown. Background fluorescence (shaded area) was assessed using an isotype control Ab (C). The bar graphs summarize the results (expressed as mean ± SD) from all analyzed subjects (D). Kruskal-Wallis H test and associated P values are indicated. *P < 0.05 and ***P < 0.0005 compared with healthy CD4+ T cells (as determined using the Mann-Whitney U test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/16/10.1182_blood-2014-08-598565/4/m_2507f2.jpeg?Expires=1769087976&Signature=132TqCwK84ONYv4OV1Ktm3nH2TDFct8lgR783i1oOPTljo9bVHgCbnmfUViTT4RSerMqRQxNv4za2pb2nz-ZOZ2VYip6oRhwbrsjpk6BlReijuA3nRp1O8WUqNLoGuR6JyIl-8-AcCwfzgkvRp64PpdvM1sUt~RbpZ2T37iUNzCPQjE6oQyrfEAruwC4FRgp4UM4t9r4abMnzvvx0nhl~s2cIozcxW-CBWA0hvWEoYak4Q~VPfy5bIhgf43~FkhSKQoZC-DpF6ZgcvT~-hnXLB6xF~TPdEX8uaP5bbKQa8vtRMGcjX7j61qX3jct13csX5f6njPRr0dOHXrDBiaItg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)