Key Points
Under physiological flow rates, plasminogen primarily accumulates on fibrin(ogen), emanating from platelets and initiates fibrinolysis.
Plasminogen is localized to defined “caps” on the surface of PS-exposing platelets in a fibrin(ogen)-dependent manner.
Abstract
The interaction of plasminogen with platelets and their localization during thrombus formation and fibrinolysis under flow are not defined. Using a novel model of whole blood thrombi, formed under flow, we examine dose-dependent fibrinolysis using fluorescence microscopy. Fibrinolysis was dependent upon flow and the balance between fibrin formation and plasminogen activation, with tissue plasminogen activator-mediated lysis being more efficient than urokinase plasminogen activator-mediated lysis. Fluorescently labeled plasminogen radiates from platelet aggregates at the base of thrombi, primarily in association with fibrin. Hirudin attenuates, but does not abolish plasminogen binding, denoting the importance of fibrin. Flow cytometry revealed that stimulation of platelets with thrombin/convulxin significantly increased the plasminogen signal associated with phosphatidylserine (PS)-exposing platelets. Binding was attenuated by tirofiban and Gly-Pro-Arg-Pro amide, confirming a role for fibrin in amplifying plasminogen binding to PS-exposing platelets. Confocal microscopy revealed direct binding of plasminogen and fibrinogen to different platelet subpopulations. Binding of plasminogen and fibrinogen co-localized with PAC-1 in the center of spread platelets. In contrast, PS-exposing platelets were PAC-1 negative, and bound plasminogen and fibrinogen in a protruding “cap.” These data show that different subpopulations of platelets harbor plasminogen by diverse mechanisms and provide an essential scaffold for the accumulation of fibrinolytic proteins that mediate fibrinolysis under flow.
Introduction
Platelet accumulation is central to the hemostatic response. Platelets are activated in vivo by numerous agonists of varying potency, including thrombin, collagen, adenosine 5′diphosphate, and thromboxane A2. Platelets exhibit a nonuniform response to activation, with distinct populations forming with different surface characteristics.1 Aggregating platelets are characterized by a spherical shape, binding of fibrinogen, and expression of the active integrin αIIbβ3, and predominantly function in clot retraction. Highly activated platelets are observed on collagen fibers1 and in the core region of a thrombus nearest the vascular injury.2 These platelets are characterized by membrane exposure of phosphatidylserine (PS), a rounded balloon-like structure, sustained increase in cytosolic Ca2+, and binding of coagulation factors.3,4 PS-exposing platelets, also termed procoagulant platelets, substantially enhance the activity of the prothrombinase complex,5,6 and subsequent thrombin and fibrin formation.7 An additional subpopulation of platelets, termed “coated” platelets, are generated in response to strong dual agonist stimulation with thrombin and collagen or convulxin (CVX).8-10 Like PS-exposing platelets, coated platelets express high levels of PS and are highly procoagulant, but are differentiated by their ability to irreversibly bind α granular proteins, such as factor V, thrombospondin, fibrinogen, fibronectin, and von Willebrand factor.11 In addition to their procoagulant functions, platelets also provide a scaffold for fibrin formation and assembly of hemostatic factors.
Platelets mediate fibrinolysis by supplying a number of fibrinolytic proteins and inhibitors, including fibrinogen, plasminogen, and plasminogen activator inhibitor-1 (PAI-1). The anti-fibrinolytic function of platelet PAI-1 has been described in vitro12,13 and in vivo.14 Like PAI-1,15 plasminogen is contained in platelet α granules16,17 and is released upon thrombin stimulation.18 Platelet-bound plasminogen supports single-chain urokinase PA (scuPA)-mediated plasma clot lysis,19 suggesting that despite being at a low concentration (0.2 nM), it is a functionally active pool. Several cell receptors for plasminogen have been identified, most of which engage lysine binding sites20 and consistent with this, can be blocked with the lysine analog εACA, as well as antibodies directed against the fibrinogen binding site on αIIbβ3.21,22 Binding of plasminogen to platelets is augmented by thrombin stimulation, suggesting exposure of additional sites on the activated membrane.21 When in association with the platelet surface, plasminogen assumes an open conformation that is more readily cleaved to plasmin19,21,23-26 and is considerably protected from inhibition by α2anti-plasmin (α2AP).27,28
Thrombi formed at high shear rates contain an abundance of platelets, whereas at low shear they are rich in erythrocytes and fibrin,29 which is aligned in the direction of flow.30 This has downstream implications in terms of thrombus stability and susceptibility to lysis29,31 and can influence penetration of cells into thrombi. Models incorporating physiological flow rates have helped define the processes governing thrombus formation.29,32-36 However, studies analyzing the impact of flow on fibrinolysis are limited and little is known about the distribution of plasminogen under these circumstances. In this study, we form thrombi from whole blood under physiological shear rates and manipulate the model to study fibrinolysis. Under flow plasminogen primarily associates with platelet-associated fibrin with a smaller pool found to be directly associated with platelets. Direct binding of plasminogen to platelets reveals differences in localization depending on the subpopulation of platelets.
Methods
Collection of blood and preparation of platelets
Blood was drawn from healthy controls according to the Declaration of Helsinki. In addition, remnant blood samples were obtained from 3 patients undergoing cardiothoracic surgery, before and after receiving 2 mg of tranexamic acid to prevent bleeding complications, as a consequence of massive dilution with crystalloids and colloids.37 Protocols were approved by the local Medical Ethical Commission (METC-13-4-084).
Peripheral blood was collected in 3.2% sodium citrate for thrombus formation or acid citrate dextrose solution A vacuettes (Greiner Bio-One Ltd) for platelet isolation. Platelets were isolated by centrifugation at 260g for 15 minutes to collect platelet-rich plasma.33 Platelet-rich plasma was centrifuged at 870g for 15 minutes and then washed by centrifugation at 870g for 15 minutes in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) wash buffer (10 mM HEPES [pH 6.6], 136 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.1% glucose, and 0.1% bovine serum albumin [BSA]) containing 0.1 U/mL apyrase (Sigma-Aldrich) and acid citrate dextrose (80 mM trisodium citrate, 52 mM citric acid, and 183 mM glucose). Pelleted platelets were resuspended in HEPES buffer (10 mM HEPES [pH 7.45], 136 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.1% glucose, and 0.1% BSA) containing 0.1 U/mL apyrase.33 Platelet counts were performed on a Siemens ADVIA 2120i Hematology System by the Haematology department, Aberdeen Royal Infirmary.
Thrombus formation and lysis under flow conditions
Citrated whole blood was perfused (1000 s−1) over a glass coverslip coated with micro-spots of 100 ng HORM fibrillar type I collagen (Takeda Pharmaceuticals) ± 100 pM recombinant human tissue factor (TF) (Innovin; Dade Behring) in a transparent parallel-plate perfusion chamber.38 Blood ± 3 µg/mL hirudin was recalcified by co-perfusing 1:10 (v/v) with HEPES buffer (pH 7.45) containing 31.5 mM MgCl2 and 63.2 mM CaCl2 by a dual inlet mechanism. Platelets were fluorescently labeled with 0.5 µg/mL 3′,3′-dihexyloxacarbocyanine iodide (DiOC6) (AnaSpec). Fibrinogen was labeled with either Alexa Fluor (AF) 647 or Oregon Green (OG) 488 (Life Technologies, Paisley, United Kingdom) and added to whole blood at 16.7 µg/mL and 75 µg/mL, respectively.36 Where indicated, thrombi were perfused with 0.8 µM glu-plasminogen (Enzyme Research Laboratories) labeled with DyLight 633 (DL633) (Pierce; Thermo Scientific). For fibrinolysis experiments, tissue PA (tPA) (Technoclone) or urokinase PA (uPA) (National Institute for Biological Standards and Control) (0 to 75 nM) were added to the blood and thrombi formed for 7 minutes before perfusing for 8 minutes with buffer 1 (HEPES buffer, pH 7.45), at indicated shear rate. In some experiments, thrombi were perfused with whole blood ± heparin (3 µg/mL) containing tPA (75 nM). If lysis was incomplete at 8 minutes, perfusion was continued for up to 25 minutes (total time) in buffer 2 (HEPES buffer, pH 7.45 containing tPA or uPA at indicated concentrations). Fluorescence time course images and z-stacks were generated using a confocal Zeiss Live 7 laser scanning microscope 60 X/1.4 NA oil immersion objective. Spatial and temporal analysis of fluorescence changes, as well as overlap coefficients R were determined using Zeiss Live 7 software and Image J (open source).
Plasmin activity assays
Washed platelets (5 × 108 platelets/mL) in HEPES buffer, pH 7.45 were stimulated for 45 minutes with 100 nM thrombin + 100 ng/mL CVX (Pentapharm or purified to homogeneity from the venom of Crotalus durissus terrificus39 ) or 15 µM thrombin receptor activator peptide 6 (TRAP-6) (Sigma-Aldrich) + CVX in the presence of 2 mM CaCl2.40 Following activation, 6 × 107 platelets/mL were removed and 0.3 µg/mL hirudin added to prevent substrate cleavage by thrombin. Plasmin generation was measured ± 1 nM tPA or uPA using 0.35 mM D-Val-Leu-Lys-7-amido-4-methylcoumarin (Sigma-Aldrich). Fluorescence release (excitation 360/40 nm, emission 460/40 nm) was detected by continuous measurement in a BioTek FLx800 fluorescence microplate reader at 37°C.
Flow cytometry
Washed platelets at 2 × 108 platelets/mL in HEPES buffer, pH 7.45, were stimulated in the presence of 2 mM CaCl2 with 100 nM thrombin ± 100 ng/mL CVX, or 15 µM TRAP-6 and 100 ng/mL CVX ± 1 µg/mL tirofiban, or 5 mM Gly-Pro-Arg-Pro amide (GPRP) (Sigma-Aldrich), or Gly-Pro-Pro-Pro (Severn Biotech Ltd) as a negative control. After 40 minutes, 0.27 µM plasminogen-DL633 was added before the addition of Annexin A5-fluorescein isothiocyanate (FITC) (1/20) (BD Biosciences). Binding of plasminogen-DL633 was measured using an LSR II flow cytometer (Becton Dickinson). A minimum of 10 000 events were collected. Data analysis was performed using FloJo software (Tree Star Inc.).
Fluorescent confocal microscopy
Washed platelets at 0.5 × 108 platelets/mL in HEPES buffer, pH 7.45 (1% BSA) were adhered to µ-Ibidi I0.4 coated with 0.6 µg equine tendon type I collagen (American Biochemical Pharmaceuticals), ±3 pmol thrombin or 0.45 nmol TRAP-6, and ±1 µg/mL tirofiban or 5 mM GPRP. In some cases, rabbit anti-human PAI-1 antibody41 labeled with DyLight 488 (1/20 dilution) or mouse anti-human monoclonal PAC-1 FITC antibody (1/20 dilution) (Becton Dickinson) were included. After stimulation for 40 minutes, 0.8 µM plasminogen-DL633 or 16.7 µg/mL fibrinogen-AF647 were added. Where indicated, Annexin A5–FITC (1/20 dilution) or Annexin A5–AF647 (1/20 dilution) (Life Technologies) and 2 mM CaCl2 were included. Images were recorded on Zeiss 710 laser scanning confocal microscope with a ×63 1.40 oil immersion objective using Zeiss Zen 2012 software. Analysis was performed on Bitplane’s Imaris ×64 software.
Statistical analysis
Statistical analysis was performed in GraphPad Prism 5.04 using one-way analysis of variance with Bonferroni post hoc test with the exception of the thrombus flow experiments, which were analyzed using Student t test (two-tailed). P < .05 was considered to be significant. Results are represented by the mean ± standard deviation (SD) or ± standard error of the mean (SEM).
Results
Visualization of fibrinolysis under high-shear flow conditions
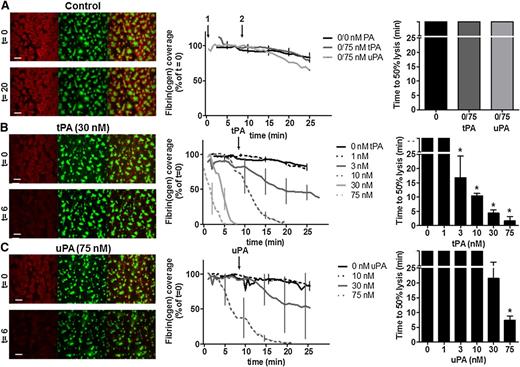
A whole blood flow model was developed to visualize fibrinolysis under a physiological shear rate (1000 s−1). Thrombi consisting of platelets and fibrin(ogen) were formed for 7 minutes on collagen + TF micro-spot surfaces ± tPA or uPA (0 to 75 nM). Fibrin formation was monitored by fluorescence microscopy as accumulation of fibrin(ogen)-AF647 (supplemental Video 1, available on the Blood Web site). Thrombi (non-occlusive) were subsequently perfused with buffer to monitor lysis in real time. In the absence of PA fibrin(ogen), coverage remained relatively unchanged for the duration of the experiment (Figure 1A). Dose-dependent fibrinolysis was visualized in thrombi containing either tPA or uPA (Figure 1B-C and supplemental Video 2). Comparable 50% lysis times were achieved with 30 nM tPA and 75 nM uPA (4.5 ± 0.6 minutes and 7.3 ± 1.5 minutes, respectively (Figure 1B-C). The lower tPA concentration required was consistent with its known fibrin specificity. Analysis of effluents, collected during tPA-mediated fibrinolysis, revealed substantial plasmin activity at 75 nM but minimal activity at 10 nM or in its absence (supplemental Figure 1A). The addition of PAs to thrombi postformation (Figure 1A) did not achieve lysis within the experimental time frame (up to 25 minutes). Interestingly, maximum fibrinogen coverage postthrombus formation was reduced in the presence of 75 nM tPA (51% ± 3% vs 75% ± 1% in the absence of tPA), reflecting concurrent fibrinolysis during thrombus formation. The change in fibrinogen coverage could not be explained by fibrinogenolysis, as minimal degradation was observed following incubation of tPA in plasma (supplemental Figure 1B). In contrast, surface coverage of DiOC6-labeled platelets was unaltered by tPA or uPA (supplemental Figure 1C-D). Furthermore, DiOC6-labeled platelet volume was investigated using confocal z-stacks (16-bit images of 1024 × 1024 pixels; 336 × 336 μm; stack distance 0.5 μm; 100 slices).42 No difference was noted in total platelet volume ± 75 nM tPA (311 μm3 ± 145 μm3 vs 267 μm3 ± 115 μm3 per field, P = .43, n = 4).
Concentration-dependent effect of plasminogen activators on fibrin degradation of thrombi under flow. Platelet-fibrin thrombi were formed by perfusion (1000 s−1) of whole blood for 7 minutes over collagen/TF in the absence or presence of tPA or uPA (0 nM to 75 nM). Blood samples were pre-incubated with DiOC6 (0.5 µg/ml) to label platelets and fibrinogen-AF647 (16.7 µg/ml). The fluorescent thrombi were perfused (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 8 minutes, followed by buffer 2 (HEPES buffer, pH 7.45 with the indicated concentration of tPA or uPA) for up to 28 minutes. Shown are representative images of thrombi labeled for fibrin(ogen) (red), platelets (green), and label overlay (yellow) (left panels). Scale bars represent 50 µm. Also shown is dose-dependent fibrinolysis in time, expressed as fibrin(ogen)-AF647 surface coverage (% of t = 0, ± SEM) (middle panels), and time to 50% lysis (right panels). (A) Absence of plasminogen activators, light bars represent controls where 75 nM tPA or uPA were present in buffer 2 only and not during thrombus formation. (B) tPA-mediated fibrinolysis and (C) uPA-mediated fibrinolysis. Data represent mean ± SEM, *P < .05 vs no tPA or uPA, n ≥ 3.
Concentration-dependent effect of plasminogen activators on fibrin degradation of thrombi under flow. Platelet-fibrin thrombi were formed by perfusion (1000 s−1) of whole blood for 7 minutes over collagen/TF in the absence or presence of tPA or uPA (0 nM to 75 nM). Blood samples were pre-incubated with DiOC6 (0.5 µg/ml) to label platelets and fibrinogen-AF647 (16.7 µg/ml). The fluorescent thrombi were perfused (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 8 minutes, followed by buffer 2 (HEPES buffer, pH 7.45 with the indicated concentration of tPA or uPA) for up to 28 minutes. Shown are representative images of thrombi labeled for fibrin(ogen) (red), platelets (green), and label overlay (yellow) (left panels). Scale bars represent 50 µm. Also shown is dose-dependent fibrinolysis in time, expressed as fibrin(ogen)-AF647 surface coverage (% of t = 0, ± SEM) (middle panels), and time to 50% lysis (right panels). (A) Absence of plasminogen activators, light bars represent controls where 75 nM tPA or uPA were present in buffer 2 only and not during thrombus formation. (B) tPA-mediated fibrinolysis and (C) uPA-mediated fibrinolysis. Data represent mean ± SEM, *P < .05 vs no tPA or uPA, n ≥ 3.
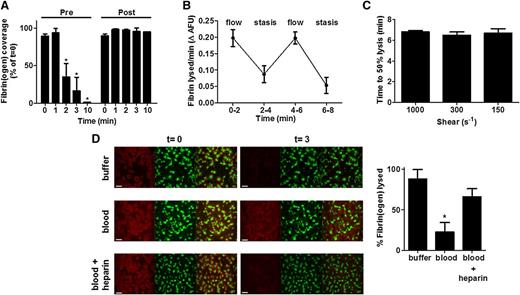
Differences in fibrinolysis were noted in cardiothoracic surgery patients treated with 2 mg of tranexamic acid, a lysine analog that inhibits plasminogen activation. Thrombi formed prior to tranexamic acid infusion lysed to a similar degree as healthy controls (75 nM tPA; 50% lysis times were 1.5 ± 0.3 minutes vs 1.75 ± 1.5 minutes, respectively). However, thrombi formed posttreatment did not show any appreciable lysis during the time frame (Figure 2A).
Fibrinolysis under physiological flow conditions. (A) Thrombi were formed from blood samples obtained from cardiothoracic patients pre- and post-tranexamic acid treatment (2 mg). Blood was preincubated with DiOC6 (0.5 µg/ml) to label platelets and fibrinogen-AF647 (16.7 µg/ml), and perfused (1000 s−1) over a collagen/TF-coated surface for 7 minutes in the presence of 75 nM tPA. HEPES buffer, pH 7.45 (t = 0) was then allowed to perfuse through thrombi at 1000 s−1. The fluorescent thrombi were lysed by perfusion (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 10 minutes. Quantification of surface area covered with fibrin(ogen) as compared with t = 0. Data represent mean ± SD, n = 3. (B) Thrombi from blood of normal individuals were formed as described for (A) except with inclusion of 10 nM tPA, and during the lysis stage flow was alternated between 1000 s−1 and stasis every 2 minutes. (C) Thrombi were formed as for (B) before perfusing at 1000 s−1, 300 s−1, or 150 s−1 (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 8 minutes, followed by buffer 2 (HEPES buffer, pH 7.45 with 10 nM tPA) until lysis was complete. Quantification is shown as time to 50% lysis (mean ± SD). (D) Thrombi were formed as above in the presence of 75 nM tPA. After formation (t = 0), thrombi were subsequently perfused with HEPES buffer, pH 7.45, whole blood, or heparinized whole blood containing tPA (75 nM) for up to 20 minutes. Representative images of thrombi labeled for fibrin(ogen) (red), platelets (green), and overlay (yellow). Scale bars represent 50 µm. Quantification shown of percentage of fibrin(ogen) lysed based on initial surface coverage from 0 to 3 minutes. AFU, average fluorescence units. Data represent mean ± SEM, n ≥ 3, P < .05.
Fibrinolysis under physiological flow conditions. (A) Thrombi were formed from blood samples obtained from cardiothoracic patients pre- and post-tranexamic acid treatment (2 mg). Blood was preincubated with DiOC6 (0.5 µg/ml) to label platelets and fibrinogen-AF647 (16.7 µg/ml), and perfused (1000 s−1) over a collagen/TF-coated surface for 7 minutes in the presence of 75 nM tPA. HEPES buffer, pH 7.45 (t = 0) was then allowed to perfuse through thrombi at 1000 s−1. The fluorescent thrombi were lysed by perfusion (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 10 minutes. Quantification of surface area covered with fibrin(ogen) as compared with t = 0. Data represent mean ± SD, n = 3. (B) Thrombi from blood of normal individuals were formed as described for (A) except with inclusion of 10 nM tPA, and during the lysis stage flow was alternated between 1000 s−1 and stasis every 2 minutes. (C) Thrombi were formed as for (B) before perfusing at 1000 s−1, 300 s−1, or 150 s−1 (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 8 minutes, followed by buffer 2 (HEPES buffer, pH 7.45 with 10 nM tPA) until lysis was complete. Quantification is shown as time to 50% lysis (mean ± SD). (D) Thrombi were formed as above in the presence of 75 nM tPA. After formation (t = 0), thrombi were subsequently perfused with HEPES buffer, pH 7.45, whole blood, or heparinized whole blood containing tPA (75 nM) for up to 20 minutes. Representative images of thrombi labeled for fibrin(ogen) (red), platelets (green), and overlay (yellow). Scale bars represent 50 µm. Quantification shown of percentage of fibrin(ogen) lysed based on initial surface coverage from 0 to 3 minutes. AFU, average fluorescence units. Data represent mean ± SEM, n ≥ 3, P < .05.
The impact of flow on tPA-mediated lysis of thrombi was investigated by alternating between a high shear rate of 1000 s−1 and stasis conditions. Lysis, quantified as change in fibrin(ogen) fluorescence over time, was reduced twofold during stasis (Figure 2B), reflecting the contribution of flow to fibrinolysis. When shear rates were altered during fibrinolysis, no differences in time to 50% lysis were observed (Figure 2C), suggesting that flow itself is the essential parameter rather than shear rate.
The described model was manipulated to visualize both thrombus formation and lysis in the presence of whole blood. Thrombi were perfused with buffer or recalcified citrated-whole blood containing 75 nM tPA. Complete lysis was visualized at around 3 minutes with 75 nM tPA in buffer, but was substantially delayed in whole blood (Figure 2D). Perfusing thrombi with heparinized blood containing 75 nM tPA achieved a comparable level of lysis to that observed in buffer (Figure 2D). These data indicate that in the presence of an anticoagulant to inhibit ongoing thrombin generation,43 and thus fibrin formation, fibrinolysis is more efficient.
Localization of plasminogen within thrombi
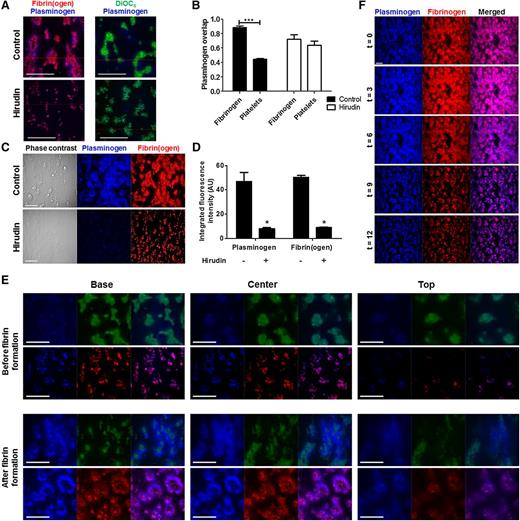
Plasminogen activation and fibrinolysis are inextricably linked. We therefore investigated the distribution of plasminogen within thrombi under physiological flow rates. Two pools of plasminogen were detected (Figure 3A); the larger pool primarily co-localized with platelet-associated fibrin(ogen), whereas a smaller pool was directly associated with platelets. Overlap coefficients (R) were high (0.89 ± 0.02) for plasminogen and fibrinogen, and lower (0.45 ± 0.01) for plasminogen and platelets (Figure 3B). Inclusion of hirudin reduced fibrin(ogen) surface area coverage from 50% ± 3% to 9% ± 1% (P < .05; Figure 3C-D). Consequently, plasminogen binding decreased 83% (P < .05; Figure 3D), and overlap coefficients for plasminogen with platelets or fibrinogen were similar under these conditions (0.64 ± 0.06 and 0.72 ± 0.07, respectively) (Figure 3B).
Plasminogen localization within thrombi. Thrombi were formed by whole blood perfusion (1000 s−1) over a collagen/TF-coated surface with or without hirudin (3 µg/ml). Platelets labeled with DiOC6 (0.5 µg/ml) or fibrinogen-OG488 (75 µg/ml) was included. Thrombi were perfused with plasminogen–DL-633 (0.8 µM). (A) Representative overlays of plasminogen (blue) and fibrin(ogen) (red), or platelets (green). (B) Overlap coefficients (R) for plasminogen with fibrin(ogen) and/or platelets as determined using Zeiss Live 7 software. (C) Representative images of thrombi labeled for plasminogen-DL633 (blue) and fibrin(ogen)-OG488 (red). (D) Quantification of plasminogen and fibrinogen fluorescence intensity (AU). (E) Confocal z-stacks were recorded of labeled thrombi (16-bit images of 1024 × 1024 pixels; 106 × 106 μm; stack distance 0.5 μm; 50 slices). Representative images and overlays of plasminogen (blue) and platelets (green), or fibrin(ogen) (red) taken from z-stacks at the base (0 μm), center (10 μm), and top (20 μm) of thrombi before (top panel) or after (bottom panel) visible fibrin formation. (F) Thrombi were formed as above after pre-incubation with plasminogen–DL-633 (0.8 µM) and fibrinogen-OG488 (75 µg/ml) in the presence of 10 nM tPA. The fluorescent thrombi were perfused (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 8 minutes, followed by buffer 2 (HEPES buffer, pH 7.45 with 10 nM tPA) for up to 28 minutes. Shown are representative images of thrombi labeled for fibrin(ogen) (red) and plasminogen (blue). Scale bars represent 50 µm. *P < .05; ***P < .001. Data represented as mean ± SEM, n ≥ 3.
Plasminogen localization within thrombi. Thrombi were formed by whole blood perfusion (1000 s−1) over a collagen/TF-coated surface with or without hirudin (3 µg/ml). Platelets labeled with DiOC6 (0.5 µg/ml) or fibrinogen-OG488 (75 µg/ml) was included. Thrombi were perfused with plasminogen–DL-633 (0.8 µM). (A) Representative overlays of plasminogen (blue) and fibrin(ogen) (red), or platelets (green). (B) Overlap coefficients (R) for plasminogen with fibrin(ogen) and/or platelets as determined using Zeiss Live 7 software. (C) Representative images of thrombi labeled for plasminogen-DL633 (blue) and fibrin(ogen)-OG488 (red). (D) Quantification of plasminogen and fibrinogen fluorescence intensity (AU). (E) Confocal z-stacks were recorded of labeled thrombi (16-bit images of 1024 × 1024 pixels; 106 × 106 μm; stack distance 0.5 μm; 50 slices). Representative images and overlays of plasminogen (blue) and platelets (green), or fibrin(ogen) (red) taken from z-stacks at the base (0 μm), center (10 μm), and top (20 μm) of thrombi before (top panel) or after (bottom panel) visible fibrin formation. (F) Thrombi were formed as above after pre-incubation with plasminogen–DL-633 (0.8 µM) and fibrinogen-OG488 (75 µg/ml) in the presence of 10 nM tPA. The fluorescent thrombi were perfused (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 8 minutes, followed by buffer 2 (HEPES buffer, pH 7.45 with 10 nM tPA) for up to 28 minutes. Shown are representative images of thrombi labeled for fibrin(ogen) (red) and plasminogen (blue). Scale bars represent 50 µm. *P < .05; ***P < .001. Data represented as mean ± SEM, n ≥ 3.
High-resolution confocal z-stacks of thrombi42 were performed to study the localization of plasminogen during thrombus formation. An intense signal from platelet aggregates was evident at the base, consistent with deposition on the collagen/TF surface (Figure 3E). Before visible fibrin fiber formation, plasminogen was detected around platelets in the base and center z-stacks (Figure 3E). Following the onset of fibrin formation, fibrin(ogen) and plasminogen radiated from platelet aggregates at the base and extended over the platelet surface as shown by the center and top z-stacks (Figure 3E). During tPA-mediated fibrinolysis, maximal plasminogen signal was visualized at t = 3 minutes, followed by a reduction in signal as the fibrin(ogen) was degraded (Figure 3F). Despite a decrease in overall signal during fibrinolysis, the overlap coefficient for plasminogen and fibrinogen remained high (R > .95). Prolonged perfusion of thrombi with buffer did not substantially alter the plasminogen signal, except in the presence of hirudin, illustrating the role of fibrin in stabilizing the binding of plasminogen (not shown).
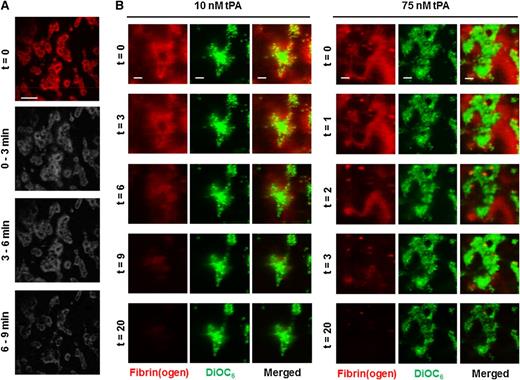
We further examined the pattern of fibrin degradation in thrombi during tPA-mediated fibrinolysis. At t = 0 minutes, the most intense fluorescent signal was directly associated with the platelet surface, indicative of dense fibrin in these areas (Figure 4A-B). Subtraction analysis revealed that platelet-associated fibrin is extremely resistant to lysis (Figure 4A). Higher magnification images of single thrombi revealed that the fibrin fibers distal to platelet aggregates were the first to be degraded (Figure 4B) (10 nM tPA, 0 to 6 minutes), whereas fibrin adjacent to platelets was the last to lyse (consistent with Figure 4A). A faster rate of lysis was observed with 75 nM tPA, but the same pattern of degradation was visualized (Figure 4B). These data indicate that tPA concentration affects the kinetics but not the pattern of fibrin degradation.
Fibrinolysis is delayed in fibrin immediately proximal to the platelet surface. Thrombi were formed by whole blood perfusion (1000 s−1) over a collagen/TF-coated surface for 7 minutes in the presence of 10 nM tPA. Thrombi were subsequently perfused (t = 0) with HEPES buffer, pH 7.45 for 8 minutes, and then HEPES buffer containing 10 nM tPA. (A) Representative image of fibrin(ogen)-AF647 staining on thrombi at the start and images subjected to subtraction analysis of fibrinogen fluorescence (n = 3). (B) Blood samples were pre-incubated with DiOC6 (0.5 µg/ml) to label platelets and fibrinogen-AF647 (16.7 µg/ml). Thrombi were allowed to form as above in the presence of 10 nM or 75 nM tPA. The fluorescent thrombi were perfused (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 8 minutes, followed by buffer 2 (HEPES buffer, pH 7.45 with indicated concentration tPA) until lysis was complete. Representative images and overlays of platelets (green) and fibrin(ogen) (red) during lysis with 10 nM or 75 nM tPA. Images are representative of n = 8. Scale bars represent 50 µm.
Fibrinolysis is delayed in fibrin immediately proximal to the platelet surface. Thrombi were formed by whole blood perfusion (1000 s−1) over a collagen/TF-coated surface for 7 minutes in the presence of 10 nM tPA. Thrombi were subsequently perfused (t = 0) with HEPES buffer, pH 7.45 for 8 minutes, and then HEPES buffer containing 10 nM tPA. (A) Representative image of fibrin(ogen)-AF647 staining on thrombi at the start and images subjected to subtraction analysis of fibrinogen fluorescence (n = 3). (B) Blood samples were pre-incubated with DiOC6 (0.5 µg/ml) to label platelets and fibrinogen-AF647 (16.7 µg/ml). Thrombi were allowed to form as above in the presence of 10 nM or 75 nM tPA. The fluorescent thrombi were perfused (t = 0) with buffer 1 (HEPES buffer, pH 7.45) for 8 minutes, followed by buffer 2 (HEPES buffer, pH 7.45 with indicated concentration tPA) until lysis was complete. Representative images and overlays of platelets (green) and fibrin(ogen) (red) during lysis with 10 nM or 75 nM tPA. Images are representative of n = 8. Scale bars represent 50 µm.
Plasminogen activation is enhanced on the stimulated platelet membrane
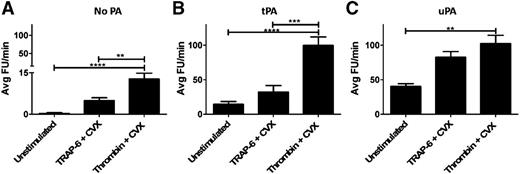
Plasmin activity was analyzed on the surface of resting platelets, or platelets stimulated with CVX plus thrombin or TRAP-6. In the absence of exogenous PA, minimal plasmin activity was detected, except when platelets were stimulated with thrombin/CVX (P < .001; Figure 5A). The addition of 1 nM tPA or uPA increased plasmin generation on unstimulated and stimulated platelets (Figure 5B-C). uPA-mediated plasmin activity was significantly augmented with thrombin/CVX and TRAP-6/CVX compared with unstimulated platelets. In contrast, tPA-mediated plasmin generation was only significantly enhanced when platelets were stimulated with thrombin/CVX. This difference may be explained by the fact that thrombin, unlike TRAP-6, cleaves fibrinogen indicating that platelet-associated fibrin supports tPA-mediated plasminogen activation.
Platelet stimulation enhances plasminogen activation. (A) Washed platelets (5 × 108 platelets/ml) were stimulated with CVX (100 ng/ml) + thrombin (100 nM) or TRAP-6 (15 µM) for 45 minutes. (B-C) Platelets were then diluted to a final concentration of 6 × 107 platelets/ml ± tPA or uPA (1 nM) in the presence of hirudin (0.3 µg/ml) and D-Val-Leu-Lys-7-amido-4-methylcoumarin (0.35 mM). Plasmin generation was measured as fluorescence release and quantified as average FU per minute. **P < .01; ***P < .001; ****P < .0001 compared with unstimulated platelets. Data are expressed as mean ± SEM, n = 5. FU, fluorescence unit.
Platelet stimulation enhances plasminogen activation. (A) Washed platelets (5 × 108 platelets/ml) were stimulated with CVX (100 ng/ml) + thrombin (100 nM) or TRAP-6 (15 µM) for 45 minutes. (B-C) Platelets were then diluted to a final concentration of 6 × 107 platelets/ml ± tPA or uPA (1 nM) in the presence of hirudin (0.3 µg/ml) and D-Val-Leu-Lys-7-amido-4-methylcoumarin (0.35 mM). Plasmin generation was measured as fluorescence release and quantified as average FU per minute. **P < .01; ***P < .001; ****P < .0001 compared with unstimulated platelets. Data are expressed as mean ± SEM, n = 5. FU, fluorescence unit.
Plasminogen binds to PS-exposing platelets
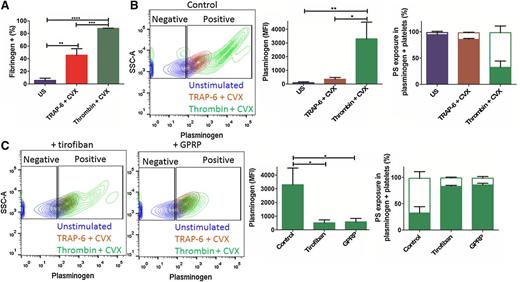
Flow cytometry was used to analyze direct binding of fibrinogen and plasminogen to washed stimulated platelets. Stimulation of platelets with TRAP-6/CVX or thrombin/CVX significantly augmented binding of fibrinogen-AF647 (P < .01 and P < .0001, respectively) (Figure 6A), with maximal binding observed with thrombin/CVX stimulation (P < .001). The percentage of unstimulated platelets that bound fibrinogen-AF647 was much lower (5.6% ± 3.5%) than the number that bound plasminogen-DL633 (29.5% ± 10.3%). However, binding of plasminogen-DL633 was still significantly enhanced by stimulation (TRAP-6/CVX, 87.2% ± 2.7%, P < .001 and thrombin/CVX, 94.5% ± 2.1%, P < .0001) (Figure 6B). A comparable number of platelets bound plasminogen-DL633 when stimulated with thrombin/CVX or TRAP-6/CVX, however, the median fluorescence intensity (MFI) was ninefold higher with thrombin/CVX stimulation. Thrombin stimulation alone generated similar numbers of plasminogen-positive platelets, consistent with previous reports,21 but MFI was reduced twofold compared with thrombin/CVX (data not shown).
Activation of platelets stimulates binding of plasminogen. Platelets (2 × 108/ml) were stimulated with thrombin (100 nM) + CVX (100 ng/ml) or TRAP-6 (15 µM) + CVX. After 40 minutes stimulation, either (A) fibrinogen-AF647 (16.7 µg/ml) or (B) plasminogen-DL633 (0.27 µM) was added for 5 minutes before the addition of Annexin A5–FITC (1/20 dilution), followed by HEPES buffer (pH 7.45) containing 2 mM CaCl2. Platelets were then analyzed by flow cytometry. (A) Percentage of fibrinogen–AF647-positive platelets. (B) Representative contour plots showing side scatter (SSC-A) against plasminogen-DL633, gated on unstained platelets (left). Plasminogen-DL633 binding quantified as MFI (middle) and the percentage of plasminogen-positive platelets that are PS-exposing (open bars) or negative (closed bars) (as indicated by Annexin A5–FITC) are shown (right). (C) Representative contour plots of thrombin/CVX-stimulated platelets in the presence of GPRP (5 mM) or tirofiban (1 µg/ml) (left). Plasminogen-DL633 binding quantified as MFI (middle) and the percentage of plasminogen-positive platelets that are PS-exposing (open bars) or negative (closed bars) are shown (right). Expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001; n = 3.
Activation of platelets stimulates binding of plasminogen. Platelets (2 × 108/ml) were stimulated with thrombin (100 nM) + CVX (100 ng/ml) or TRAP-6 (15 µM) + CVX. After 40 minutes stimulation, either (A) fibrinogen-AF647 (16.7 µg/ml) or (B) plasminogen-DL633 (0.27 µM) was added for 5 minutes before the addition of Annexin A5–FITC (1/20 dilution), followed by HEPES buffer (pH 7.45) containing 2 mM CaCl2. Platelets were then analyzed by flow cytometry. (A) Percentage of fibrinogen–AF647-positive platelets. (B) Representative contour plots showing side scatter (SSC-A) against plasminogen-DL633, gated on unstained platelets (left). Plasminogen-DL633 binding quantified as MFI (middle) and the percentage of plasminogen-positive platelets that are PS-exposing (open bars) or negative (closed bars) (as indicated by Annexin A5–FITC) are shown (right). (C) Representative contour plots of thrombin/CVX-stimulated platelets in the presence of GPRP (5 mM) or tirofiban (1 µg/ml) (left). Plasminogen-DL633 binding quantified as MFI (middle) and the percentage of plasminogen-positive platelets that are PS-exposing (open bars) or negative (closed bars) are shown (right). Expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001; n = 3.
Staining with Annexin V–FITC revealed that 66.2% ± 12.2% of the thrombin/CVX-stimulated platelets exposed PS. The majority of plasminogen-positive thrombin/CVX- stimulated platelets were PS-exposing (65.8% ± 12.6%; Figure 6B), in contrast to unstimulated and TRAP-6/CVX-stimulated platelets (3.8% ± 2.4% and 12.6% ± 1.0%, respectively). These differences reinforce a role for fibrin in plasminogen binding. Consistent with this inclusion of tirofiban, a potent inhibitor of the αIIbβ3, decreased the MFI of plasminogen-DL633 on thrombin/CVX-stimulated platelets sevenfold (P < .05) (Figure 6C), but did not change the number of positive platelets. GPRP is analogous to the amino-terminus of Aα-chain and Bβ-chain of fibrinogen and inhibits fibrin polymerization. GPRP reduced the MFI of plasminogen-DL633 in thrombin/CVX-stimulated platelets sixfold (P < .05) (Figure 6C), whereas Gly-Pro-Pro-Pro, a negative control peptide, had no effect (not shown). The change in relative amount of plasminogen bound in the presence of tirofiban or GPRP correlated with the number of PS-exposing platelets. No significant additional reduction in plasminogen binding to thrombin/CVX-stimulated platelets occurred with both tirofiban and GPRP. Neither tirofiban nor GPRP altered plasminogen binding in unstimulated or TRAP-6 + CVX-stimulated platelets (not shown). These results imply that αIIbβ3 activation and fibrin polymerization are essential for maximal binding of plasminogen to PS-exposing platelets.
Plasminogen localizes in “caps” on PS-exposing platelets
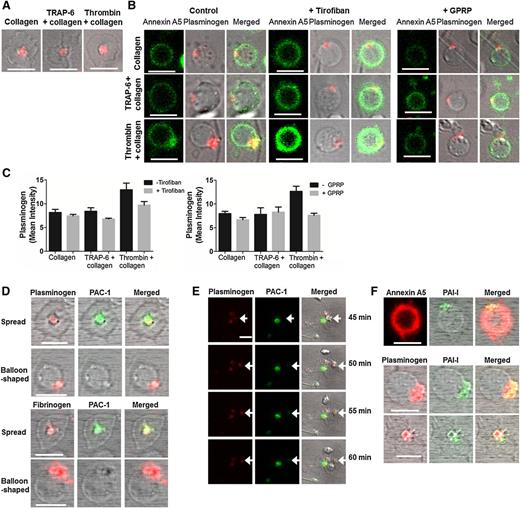
Plasminogen binding to the surface of live unpermeablized platelets was visualized by fluorescent confocal microscopy. Slides coated with collagen alone or in combination with TRAP-6 or thrombin generated 2 distinct platelet subpopulations. PS-negative platelets with a spread morphology accumulated plasminogen-DL633 in the center over the granulomere, whereas balloon-shaped PS-exposing platelets (indicated by Annexin A5–FITC binding) bound plasminogen in a protruding “cap” (Figure 7A-B). Increased plasminogen-DL633 binding to the “cap” was observed with thrombin stimulation. Tirofiban and GPRP diminished, but did not abolish, plasminogen-DL633 binding on PS-exposing platelets (Figure 7B-C), consistent with flow cytometry data. These data indicate that fibrin augments the localization of plasminogen in “caps” of PS-exposing platelets. Exogenous fibrinogen-AF647 also bound to the “cap” of balloon-shaped platelets (Figure 7D). The presence of active αIIbβ3 was analyzed using an antibody to PAC-1. PAC-1 co-localized with plasminogen and fibrinogen in the center of spread platelets but was absent from the “caps” of balloon-shaped PS-exposing platelets (Figure 7D). A time course following platelet stimulation revealed that plasminogen-DL633 was present in spread platelets prior to visualization of PAC-1, indicating that binding of plasminogen to this subpopulation is not dependent on active αIIbβ3 (Figure 7E). PAI-1 is contained in platelet α granules and is released upon stimulation.15 We found that PAI-1 co-localized with plasminogen in both the protruding “cap” of PS-exposing platelets and in spread platelets (Figure 7F). These data suggest that these “caps” serve as a focal point for localization of pro- and anti-fibrinolytic proteins.
Plasminogen localizes in “caps” of PS-exposing platelets. Platelets (0.5 × 108/ml) were adhered to a collagen (0.6 µg) ± thrombin (3 pmol) or ± TRAP-6 (0.45 µmol) coated slide ± GPRP (5 mM) or ± tirofiban (1 µg/ml). After 40 minutes incubation, plasminogen-DL633 (0.8 µM) or fibrinogen-AF647 (16.7 µg/ml) was added for 5 minutes. (A) Spread PS-negative platelets showing plasminogen-DL633 binding. (B) Plasminogen-DL633 binding on PS-positive platelets (left) ± tirofiban (middle), or GPRP (right). Annexin A5–FITC (1/20 dilution) and 2 mM CaCl2 were added immediately prior to imaging. (C) Representative quantification of plasminogen-DL633 binding expressed as mean intensity ± SEM determined using Bitplane’s Imaris ×64 software. (D) Platelets on collagen + thrombin surface stained with PAC-1 FITC showing plasminogen-DL633 (top panel) and fibrinogen-AF647 (bottom panel) binding on spread- and balloon-shaped subpopulations. (E) Platelets on collagen + thrombin surface stained with PAC-1 FITC and showing plasminogen-DL633 binding. Arrows indicate a platelet with plasminogen bound that tethers to a spread plasminogen-positive platelet, which subsequently expresses active αIIbβ3, represented by positive PAC-1 staining. Images were recorded every minute for 60 minutes and selected images are shown. (F) Staining for platelet-derived PAI-1 in collagen + thrombin-stimulated platelets using an anti–PAI-1 DyLight 488 antibody and PS (Annexin A5-AF647) (top). PAI-1 co-localization with plasminogen-DL633 in balloon-shaped (middle) and spread platelets (bottom). Scale bars represent 5 µm. Images are representative of n = 3.
Plasminogen localizes in “caps” of PS-exposing platelets. Platelets (0.5 × 108/ml) were adhered to a collagen (0.6 µg) ± thrombin (3 pmol) or ± TRAP-6 (0.45 µmol) coated slide ± GPRP (5 mM) or ± tirofiban (1 µg/ml). After 40 minutes incubation, plasminogen-DL633 (0.8 µM) or fibrinogen-AF647 (16.7 µg/ml) was added for 5 minutes. (A) Spread PS-negative platelets showing plasminogen-DL633 binding. (B) Plasminogen-DL633 binding on PS-positive platelets (left) ± tirofiban (middle), or GPRP (right). Annexin A5–FITC (1/20 dilution) and 2 mM CaCl2 were added immediately prior to imaging. (C) Representative quantification of plasminogen-DL633 binding expressed as mean intensity ± SEM determined using Bitplane’s Imaris ×64 software. (D) Platelets on collagen + thrombin surface stained with PAC-1 FITC showing plasminogen-DL633 (top panel) and fibrinogen-AF647 (bottom panel) binding on spread- and balloon-shaped subpopulations. (E) Platelets on collagen + thrombin surface stained with PAC-1 FITC and showing plasminogen-DL633 binding. Arrows indicate a platelet with plasminogen bound that tethers to a spread plasminogen-positive platelet, which subsequently expresses active αIIbβ3, represented by positive PAC-1 staining. Images were recorded every minute for 60 minutes and selected images are shown. (F) Staining for platelet-derived PAI-1 in collagen + thrombin-stimulated platelets using an anti–PAI-1 DyLight 488 antibody and PS (Annexin A5-AF647) (top). PAI-1 co-localization with plasminogen-DL633 in balloon-shaped (middle) and spread platelets (bottom). Scale bars represent 5 µm. Images are representative of n = 3.
Discussion
Knowledge of the location of plasminogen in thrombi is crucial to our understanding of initiation, regulation, and progression of fibrinolysis under physiological shear rates. In this study, we used a novel whole blood flow model and found that plasminogen largely associated with the fibrin network emanating from platelet aggregates. A smaller but significant pool directly associated with the platelet surface. We found that subpopulations of platelets showed different distributions of plasminogen, with it localized to distinct “caps” on PS-exposing platelets.
The composition of the fibrin network affects the rate of fibrinolysis, with areas of dense thin fibers being lysed more slowly.44 Fibrin also acts as a cofactor for tPA-mediated plasminogen activation, with thin fibers being less effective than thick fibers.44,45 We found that fibrinolysis was accelerated by flow, irrespective of the shear rate applied, and was dependent on the PA and its concentration. Fibrinolysis with tPA was more efficient, consistent with its binding to fibrin, which also protects it from inhibition by PAI-1.46 Partial degradation of fibrin by plasmin exposes new carboxyl-terminal lysine residues, which enhance binding of plasminogen thereby accelerating lysis.47-52 In our flow model, we visualized this phenomenon in real time as a wave of plasminogen accumulation following initiation of tPA-mediated fibrinolysis, which subsequently receded as lysis progressed. Cardiothoracic patients examined here received the lysine analog transexamic acid to prevent on-going fibrinolysis during surgery. Prior to drug administration, lysis of thrombi from these patients was similar to controls, however, following treatment, no lysis was detected during the time frame. The use of transexamic acid in this patient group exemplifies the importance of plasminogen binding to exposed lysine binding sites on partially degraded fibrin. Together, these results highlight the role of the fibrin network in orchestrating its own destruction by localizing the fibrinolytic process to its surface. We found that lysis of fibrin proximal to platelets was markedly delayed, in line with previous observations in static clots.53 The fibrin network around platelets is composed of thin densely packed fibers,44,53 which are less efficient at supporting plasminogen activation and may represent a protective mechanism to stabilize early thrombi and possibly prevent against embolization.
We showed that the majority of thrombin/CVX-stimulated platelets that bound plasminogen were PS-exposing and localized plasminogen to a distinct “cap” on their surface. Coated platelets accumulate fibrin(ogen) on their surface8,9 and Abaeva et al54 recently reported that platelet-derived fibrin(ogen) and thrombospondin are focused in a “cap” on the activated membrane. Our results indicate that exogenous fibrinogen also binds the “cap” on PS-exposing platelets co-localizing with plasminogen. Interestingly, we have recently shown that platelet-derived factor XIII-A is found in the “cap” of PS-exposing platelets.55 The presence of activated FXIII-A potentially stabilizes fibrin emanating from the platelet surface both mechanically, via cross-linking of fibrin, and against fibrinolytic degradation by cross-linking α2AP.55 Coated platelets express αIIbβ3 on their surface that becomes inactivated after occupation by a ligand such as fibrinogen.3,9,56 PS-exposing platelets in this study did not express the active αIIbβ3, as demonstrated by the lack of PAC-1 staining in the “cap.” Intriguingly, despite the lack of active integrin, we show that blocking αIIbβ3 and fibrin polymerization attenuates plasminogen binding to PS-exposing platelets. Consistent with this, platelets from patients with Glanzmann thrombasthenia who lack αIIbβ3 display reduced plasminogen binding.57 Inhibition of αIIbβ3 initiates thrombin signaling through GPIb, which requires polymerizing fibrin.58 It is unlikely that GPIb has a role in plasminogen binding, as levels on resting- and thrombin-stimulated platelets from patients with Bernard–Soulier syndrome, who lack GPIb, are similar to controls.57 We observed a significant reduction in plasminogen MFI when platelets were treated with GPRP to inhibit fibrin polymerization. Together, these data indicate that αIIbβ3 has an indirect role in plasminogen binding via fibrinogen, which is subsequently polymerized to fibrin, thereby amplifying the number of plasminogen binding sites.
Platelets contain both α2AP59 and PAI-115,60-63 that are released upon stimulation. We found that a proportion of platelet PAI-1 co-localized with plasminogen binding in PS-exposing and spread platelets. Therefore, local plasminogen activation on the platelet surface must overcome PAI-1 inhibition to generate functional plasmin. Plasmin activity was detected in effluents collected during fibrinolysis with 75 nM tPA, but not with 10 nM tPA, despite this concentration achieving lysis of thrombi. The lack of quantifiable plasmin in effluents can be explained by protection from a2AP inhibition via binding to the fibrin surface64 and the consumption of free enzyme by inhibitors. In addition to enhanced plasminogen binding to thrombin/CVX-stimulated platelets, we also showed an increase in plasmin activity, even in the absence of exogenous PAs, suggesting the presence of endogenous PA activity on platelets. Higher plasmin activity was observed in thrombin/CVX-stimulated platelets compared with TRAP-6/CVX stimulation with tPA, which can be attributed to the presence of fibrin.65 Plasmin treatment of platelets augments the number of binding sites for plasminogen in both unstimulated and adenosine 5′diphosphate-stimulated platelets,25,26 and enhances tPA activity. In contrast, plasmin generation on platelets treated with uPA was significantly augmented by TRAP-6/CVX and thrombin/CVX stimulation. Cell-bound uPA is capable of activating lys-plasminogen and εACA-liganded plasminogen, which has an open conformation similar to lys-plasminogen, without the requirement of being bound to the same cell surface.23 Indeed, a crosstalk mechanism of activation of platelet-bound plasminogen by uPA bound to monocytes or endothelial microparticles has been described,23 and our work has previously shown that scuPA-mediated fibrinolysis was enhanced by platelet-associated plasminogen.19 These reports highlight the different mechanisms by which these PAs activate plasminogen and regulate fibrinolysis.
Recently, a hierarchical thrombus structure has been observed in vivo, in which 2 discrete regions of platelet activation were observed.2 The inner core is rich in fibrin and thrombin and consists of tightly packed platelets with extensive α granule release. A loosely packed shell of platelets with minimal α granule release encases the core. Here, we demonstrate plasminogen accumulation around platelet aggregates at the “core” of the thrombus. As fibrin forms, plasminogen co-localizing with fibrin(ogen) radiates from the aggregates extending over their surface into the “loosely packed shell.” We found a high overlap coefficient for plasminogen and fibrin(ogen) with the most intense fibrin(ogen) signal in direct association with platelets. Thrombi perfused with whole blood containing heparin lysed significantly faster than whole blood alone, highlighting the importance of on-going fibrin formation in these thrombi as a result of procoagulant activity. Notable work by Stalker et al66 has recently shown that integrin αIIbβ3 outside-in signaling localizes thrombin activity within the core region of thrombi by minimizing solute transport. Our studies on plasminogen localization and plasmin activity suggest that it may be regulated by similar mechanisms via the interaction of fibrin(ogen) with αIIbβ3 within the microenvironment of the thrombus.
In conclusion, a functional pool of plasminogen accumulates on the platelet membrane following strong agonist stimulation. Under physiological flow conditions, plasminogen binds directly to platelets but is predominantly visualized on fibrin(ogen) radiating from PS-exposing platelets, and it is this pool that fluctuates during fibrinolysis. Two subpopulations of platelets were observed with distinct plasminogen and fibrinogen binding characteristics. Spread platelets accumulate plasminogen and fibrinogen centrally over the granulomere via a αIIbβ3-dependent manner. In contrast, PS-exposing platelets, such as those found in the core of a thrombus bind plasminogen to protruding “caps” via platelet-derived fibrin(ogen) and display an enhanced capacity to generate plasmin on their surface. These data suggest a role for PS-exposing platelets in modulating local fibrinolysis under physiological flow conditions within the microenvironment of the thrombus. Interestingly, a recent report described thrombolysis with scuPA fused to single-chain antibody fragments, which binds activated αIIbβ3, in a plasminogen-dependent manner.67 Targeting this sub-population of platelets in thrombi could prove useful in the development of novel thrombolytics to augment local fibrinolysis.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Microscopy and Histology Core Facility and the Iain Fraser Cytometry Centre at the University of Aberdeen for excellent advice and use of the facilities.
This work was supported by grants from the British Heart Foundation (FS/11/2/28579) (N.J.M., A.S.L.) and (PG/11/1/28461) (N.J.M., C.S.W.), the National Health Service Grampian Endowment (grant 14/43) (C.S.W., N.J.M.), Friends of Anchor (N.J.M.), the Landsteiner Foundation for Blood Transfusion Research (1006) (F.S., P.E.J.v.d.M., J.W.M.H.), and the Cardiovascular Centre Maastricht (F.S., P.E.J.v.d.M., J.W.M.H.). Travel for this project was supported by a grant from the British Society for Haemostasis and Thrombosis (N.J.M., C.S.W.).
Authorship
Contribution: C.S.W. and F.S. performed the research, analyzed the data, and wrote the manuscript; T.G.M. and A.S.L. performed the research; M.D.L. contributed vital patient samples; P.E.J.v.d.M. supervised the research; and J.W.M.H. and N.J.M. supervised the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicola J. Mutch, School of Medicine and Dentistry, Institute of Medical Sciences, Foresterhill, University of Aberdeen, Aberdeen AB25 2ZD, United Kingdom; e-mail: n.j.mutch@abdn.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal