Key Points
GMI-1070 led to statistically insignificant but large reductions in time to resolution of VOC and a significant reduction in opioid use.
These results support a role for selectins in VOC and progression to a phase 3 study of GMI-1070 for SCD patients with vaso-occlusion.
Abstract
Treatment of vaso-occlusive crises (VOC) or events in sickle cell disease (SCD) remains limited to symptom relief with opioids. Animal models support the effectiveness of the pan-selectin inhibitor GMI-1070 in reducing selectin-mediated cell adhesion and abrogating VOC. We studied GMI-1070 in a prospective multicenter, randomized, placebo-controlled, double-blind, phase 2 study of 76 SCD patients with VOC. Study drug (GMI-1070 or placebo) was given every 12 hours for up to 15 doses. Other treatment was per institutional standard of care. All subjects reached the composite primary end point of resolution of VOC. Although time to reach the composite primary end point was not statistically different between the groups, clinically meaningful reductions in mean and median times to VOC resolution of 41 and 63 hours (28% and 48%, P = .19 for both) were observed in the active treatment group vs the placebo group. As a secondary end point, GMI-1070 appeared safe in acute vaso-occlusion, and adverse events were not different in the two arms. Also in secondary analyses, mean cumulative IV opioid analgesic use was reduced by 83% with GMI-1070 vs placebo (P = .010). These results support a phase 3 study of GMI-1070 (now rivipansel) for SCD VOC. This trial was registered at www.clinicaltrials.gov as #NCT01119833.
Introduction
Sickle cell disease (SCD) is the most common hemoglobinopathy, affecting between 90 000 and 100 000 Americans1 and ∼13 million people worldwide.2,3 In the United States alone, SCD is responsible for >75 000 hospitalizations and >$1 billion in medical expenditures annually.4
Sickle hemoglobin (HbS) results from the substitution of valine for glutamic acid in the β-globin chain. Homozygosity for the gene encoding HbS, as well as compound heterozygosity for one HbS gene and one nonfunctioning thalassemic β-globin gene, result in the most severe forms of SCD. Vaso-occlusive crises (VOC) are the most common disease manifestation and account for the vast majority of hospitalizations.5 Hydroxyurea (HU), the only drug approved for SCD, decreases the frequency of but does not eliminate VOC and acute chest syndrome (ACS).6 Once VOC occurs, there remains no specific therapy to treat the underlying pathophysiology.
Sickle red cells and leukocytes adhere to endothelium in low shear circulations, such as postcapillary venules.7-14 Binding of both red cells and leukocytes to endothelial selectins has been proposed to have a critical role in SCD VOC.13,15-17 Endothelial E-selectin expression is upregulated by inflammatory cytokines and can become a target, along with P-selectin, for leukocyte adhesion.15,16 Cytokine-stimulated VOC in sickle transgenic mice is inhibited when mice are deficient in P- and E-selectins.16 Administration of the pan-selectin inhibitor GMI-1070 during VOC in sickle mice reduced adhesion of leukocytes and capture of sickle red cells by leukocytes, thus improving VOC and survival.18 Therefore, we aimed to evaluate the efficacy of GMI-1070 in hospitalized patients with VOC.
Methods
Study design and conduct
This prospective, randomized, placebo-controlled, multicenter, double-blind, adaptive phase 2 study (#NCT01119833) of the novel pan-selectin inhibitor GMI-1070 enrolled SCD patients requiring hospitalization for the treatment of VOC. The single primary efficacy end point was time to resolution of VOC, defined as when any one of the following occurred: sustained 1.5 cm decrease in visual analog scale (VAS) pain score from baseline19,20 and transition to oral analgesia; or documentation in the patient chart that both patient and physician agreed that the patient was ready for hospital discharge; or written order for discharge. Secondary end points included length of stay (LOS), cumulative opioid use (morphine equivalent units [MEU]/kg), safety, and pharmacokinetics. Sample size calculations were based on the assumption that variances would be the same in both treatment groups and that GMI-1070 would decrease time to VOC resolution by 40%. Enrollment of 76 subjects was thereby calculated to achieve 83% power with a twosided α of .045. Sites with pediatric and adult sickle cell practices were included to encourage enrollment of all ages in the study.
The study was performed under an Investigational New Drug (United States) and Clinical Trial Application (Canada), in compliance with all regulatory requirements. Twenty-two sites participated in the study, 17 of which enrolled subjects. All sites obtained approval from Institutional Review Boards. The full protocol is available as supplemental Material on the Blood Web site.
Patients
Eligibility criteria included history of homozygous hemoglobin S (HbSS) or HbSβ0 thalassemia and requirement for hospital admission and parenteral narcotics for treatment of uncomplicated VOC, defined as a painful episode without other apparent causes of pain and without significant organ dysfunction or signs or symptoms of systemic infection, including fever > 39°C. The study protocol, including inclusion and exclusion criteria, was altered 4 times to extend the age range to 12 to 60 years, enhance enrollment by allowing a history of more frequent admissions for VOC and more recent transfusion, and adjust study drug dose per interim pharmacokinetic (PK) analyses (Study Protocol; supplemental Figure 1). After all amendments, patients were eligible for the study if they had had 5 or fewer episodes of VOC in the last 6 months, had not been transfused in the prior 14 days, and were 12 to 60 years old. Exclusion for renal dysfunction, ACS, major infection, and other major medical conditions and organ dysfunction remained unchanged.
Study therapy
Study drug (GMI-1070 or placebo, randomized 1:1, stratified by site in blocks of four) was IV administered. Initially, a loading dose of study drug 20 mg/kg was given to achieve target plasma levels, followed by ≤14 subsequent 10 mg/kg doses every 12 hours to achieve minimum plasma levels > 20 μg/mL, based on phase 1 steady state data.21 After preplanned population PK analyses in the first 11 subjects and procurement of Institutional Review Board approval, doses were doubled, per protocol, to reach the targeted plasma nadir concentration. Additional prospectively planned PK analyses confirmed that the higher dose level achieved the targeted plasma nadir drug concentration. All other treatments of VOC were per institutional standard of care and provider discretion, including opioid therapy, use of non-opioid analgesics, hydration, antibiotics, transfusion, and timing and method of transition to oral analgesia.
Efficacy and safety assessments
The primary outcome was time from start of study medication to time at which the subject met criteria for resolution of VOC, as defined above. VAS pain scores were recorded every 4 hours while subjects were awake. Safety was assessed by monitoring physical examinations, routine laboratory values, pain scores, and adverse events (AEs). The latter were defined per International Conference on Harmonization, Section E2A, as untoward medical occurrences or clinically significant abnormal laboratory changes occurring during or after study treatment. Serious AEs (SAEs) were those AEs that were life-threatening or resulted in or prolonged hospitalization. ACS was defined as a new infiltrate on chest radiograph, accompanied by fever >39°C, hypoxia, chest pain, or suspicious findings on respiratory examination.
Statistical analysis
All subjects receiving at least 1 dose of study drug were included in analyses of results (modified intent-to-treat). Time to resolution of VOC was recorded for all subjects. For the primary outcome of the study, differences in time to resolution of VOC by treatment (pooled GMI-1070 vs placebo) were analyzed using the F-test from an analysis of covariance (ANCOVA) model, adjusting for sex and age (continuous). Individual components of the primary composite end point were also evaluated by this method, as secondary analyses. Least squares (LS) mean comparison by ANCOVA adjusted for covariates was used as the primary analysis method because there was no censoring with this composite end point, and thus normal distribution could be assumed; further, it allowed for controlling for possible confounders, the most likely of which were age and sex. Time to resolution of VOC and individual components of the primary end point were also compared by Kaplan-Meier estimates and log-rank test. This latter analysis was used as a secondary nonparametric approach to understand and minimize the impact of potential outliers. In a phase 2 trial evaluating efficacy while also assessing the utility of various end points, the use of both methods helps elucidate the range, variability, effect size, impact of outliers, and impact of skewed distribution to inform planning for phase 3.
Total hourly opioid received was standardized using MEU/kg and analyzed using a linear mixed model, adjusted for age and sex. Frequency of VOC complications, including rehospitalization for recurrent VOC within 14 and 30 days, was compared using Fisher’s exact test. P values for secondary end points were meant to be descriptive; therefore, no adjustment for multiple testing was made. Where P values are not presented, formal comparisons were not made.
Results
Patient and treatment characteristics
Eighty-one subjects were consented and randomized and 76 subjects were dosed (56 adults and 20 children) between May 2010 and December 2012 (Figure 1). Those no longer meeting criteria after randomization were excluded for rising creatinine, pain resolution, and lack of venous access. Baseline characteristics were similar between the GMI-1070 and placebo groups (Table 1), although subjects receiving placebo were heavier and more frequently had a history of pulmonary hypertension. Thirty-one subjects (18 GMI-1070, 13 placebo; 21 adults, 10 children) were enrolled at the original dose regimen and 45 subjects (25 GMI-1070, 20 placebo; 35 adults, 10 children) at the higher dose regimen.
Baseline subject demographic and clinical characteristics
| . | GMI-1070 (N = 43) . | Placebo (N = 33) . | P . |
|---|---|---|---|
| Characteristic | |||
| Male sex, N (%) | 18 (41.90) | 13 (39.40) | .829 |
| Age (y) | |||
| Mean (SD) | 25.4 (10.83) | 25.0 (10.20) | .855 |
| Median (range) | 23.0 (12, 50) | 22.0 (14, 51) | |
| Weight (kg) | |||
| Mean (SD) | 61.7 (17.01) | 69.9 (15.66) | .034 |
| Median (range) | 58.7 (32, 109) | 69.1 (46, 113) | |
| BMI | |||
| Mean (SD) | 22.7 (5.05) | 24.5 (5.50) | .152 |
| Median (range) | 21.5 (15, 41) | 23.2 (18, 42) | |
| Hemoglobin diagnosis | |||
| HbSS | 39 | 30 | .144 |
| Hb Sβ0 thalassemia | 1 | 3 | |
| HbSC | 3* | 0 | |
| Medical history | |||
| HU therapy, N (%) | 22 (51.2) | 23 (69.7) | .106 |
| Daily out-patient analgesics, N (%) | 18 (41.9) | 19 (57.6) | .177 |
| Admission for VOC, N (%) | |||
| In previous 12 mo | 33 (76.7) | 25 (75.8) | .921 |
| ≥3 episodes in previous 12 mo | 13 (30.2) | 14 (42.4) | .274 |
| ACS, N (%) | |||
| In previous 12 mo | 5 (11.6) | 6 (18.2) | .517 |
| History of avascular necrosis, N (%) | 15 (34.9) | 8 (24.2) | .320 |
| History of pulmonary hypertension, N (%) | 3 (7.0) | 9 (27.3) | .017 |
| Admission values | |||
| VAS at presentation | |||
| Mean (SD) | 8.28 (1.57) | 8.97 (1.52) | .079 |
| Median (range) | 8.0 (5, 10) | 10.0 (5, 10) | |
| Hemoglobin, g/dL | |||
| Mean (SD) | 8.32 (1.40) | 8.41 (1.57) | .745 |
| Median (range) | 8.60 (5.5, 11.7) | 8.30 (5.4, 13.9) | |
| WBC count, ×103/μL | |||
| Mean (SD) | 13.01 (5.09) | 13.78 (5.66) | .539 |
| Median (range) | 12.08 (3.90, 28.30) | 12.70 (4.31, 29.00) | |
| Absolute neutrophil count, ×103/μL | |||
| Mean (SD) | 7.41 (3.87) | 8.38 (5.16) | .389 |
| Median (range) | 6.60 (1.70, 15.97) | 7.32 (2.30, 26.77) | |
| Baseline hemoglobin A (N = 30) | |||
| <25%, N (%) | 12 (27.9) | 14 (42.4) | >.999 |
| 25 to <50%, N (%) | 1 (2.3) | 2 (6.1) | |
| >50 to <75%, N (%) | 0 (0) | 1 (3.0) |
| . | GMI-1070 (N = 43) . | Placebo (N = 33) . | P . |
|---|---|---|---|
| Characteristic | |||
| Male sex, N (%) | 18 (41.90) | 13 (39.40) | .829 |
| Age (y) | |||
| Mean (SD) | 25.4 (10.83) | 25.0 (10.20) | .855 |
| Median (range) | 23.0 (12, 50) | 22.0 (14, 51) | |
| Weight (kg) | |||
| Mean (SD) | 61.7 (17.01) | 69.9 (15.66) | .034 |
| Median (range) | 58.7 (32, 109) | 69.1 (46, 113) | |
| BMI | |||
| Mean (SD) | 22.7 (5.05) | 24.5 (5.50) | .152 |
| Median (range) | 21.5 (15, 41) | 23.2 (18, 42) | |
| Hemoglobin diagnosis | |||
| HbSS | 39 | 30 | .144 |
| Hb Sβ0 thalassemia | 1 | 3 | |
| HbSC | 3* | 0 | |
| Medical history | |||
| HU therapy, N (%) | 22 (51.2) | 23 (69.7) | .106 |
| Daily out-patient analgesics, N (%) | 18 (41.9) | 19 (57.6) | .177 |
| Admission for VOC, N (%) | |||
| In previous 12 mo | 33 (76.7) | 25 (75.8) | .921 |
| ≥3 episodes in previous 12 mo | 13 (30.2) | 14 (42.4) | .274 |
| ACS, N (%) | |||
| In previous 12 mo | 5 (11.6) | 6 (18.2) | .517 |
| History of avascular necrosis, N (%) | 15 (34.9) | 8 (24.2) | .320 |
| History of pulmonary hypertension, N (%) | 3 (7.0) | 9 (27.3) | .017 |
| Admission values | |||
| VAS at presentation | |||
| Mean (SD) | 8.28 (1.57) | 8.97 (1.52) | .079 |
| Median (range) | 8.0 (5, 10) | 10.0 (5, 10) | |
| Hemoglobin, g/dL | |||
| Mean (SD) | 8.32 (1.40) | 8.41 (1.57) | .745 |
| Median (range) | 8.60 (5.5, 11.7) | 8.30 (5.4, 13.9) | |
| WBC count, ×103/μL | |||
| Mean (SD) | 13.01 (5.09) | 13.78 (5.66) | .539 |
| Median (range) | 12.08 (3.90, 28.30) | 12.70 (4.31, 29.00) | |
| Absolute neutrophil count, ×103/μL | |||
| Mean (SD) | 7.41 (3.87) | 8.38 (5.16) | .389 |
| Median (range) | 6.60 (1.70, 15.97) | 7.32 (2.30, 26.77) | |
| Baseline hemoglobin A (N = 30) | |||
| <25%, N (%) | 12 (27.9) | 14 (42.4) | >.999 |
| 25 to <50%, N (%) | 1 (2.3) | 2 (6.1) | |
| >50 to <75%, N (%) | 0 (0) | 1 (3.0) |
BMI, body mass index; WBC, white blood cells.
Subjects enrolled with historical documentation of HbSS but found on Hb electrophoresis to have HbSC.
All subjects received first study drug dose within 24 hours of initial evaluation (Table 2). Fifty-eight subjects continued study drug until resolution of VOC or they had received the maximum 15 doses; subjects on GMI-1070 received a mean (standard deviation [SD]) of 6.4 (4.8) doses, whereas those on placebo received 8.2 (5.0). Reasons for study drug discontinuation are shown in Table 2. AEs causing discontinuation of study drug included ACS (5/43 and 3/33 subjects, GMI-1070 and placebo, respectively), fever alone (2/43 and 1/33 subjects, respectively), and fever with thrombocytopenia (1/43 receiving GMI-1070); the latter subject had a prior history of thrombocytopenia and developed grade 3 thrombocytopenia unrelated to study drug. That subject also developed mild neutropenia. These findings were thought to result from a viral infection and study drug was discontinued. The majority (67%) of subjects who discontinued study drug due to AEs did so for events occurring early, prior to dose 4.
Treatment and outcomes
| . | GMI-1070 . | Placebo . | P . |
|---|---|---|---|
| First medical evaluation to treatment (h) | |||
| N | 43 | 33 | |
| Mean (SD)* | 14.85 (4.36) | 15.35 (5.33) | |
| LS mean (SE)† | 14.54 (0.72) | 15.37 (0.82) | .445 |
| Median (CI)‡ | 14.7 (13.1, 16.6) | 15.2 (12.5, 17.3) | .457 |
| Reason for discontinuation of study drug, N (%) | |||
| N | 43 | 33 | .288§ |
| Resolution of VOC | 28 (65.1) | 19 (57.6) | |
| Received maximum (15 doses) | 6 (14) | 5 (15.2) | |
| No improvement on day 5 | 1 (2.3) | 2 (6.1) | |
| AE | 8 (18.6) | 4 (12.1) | |
| Other | 0 (0) | 3 (9.1) | |
| End point component first achieved, N (%) | |||
| N | 43 | 33 | |
| Sustained decrease in VAS score and transition to oral analgesia | 19 (44.2) | 15 (45.5) | |
| Agreement about readiness for discharge | 18 (41.9) | 16 (48.5) | |
| Hospital discharge | 8 (18.6) | 4 (12.1) | |
| Time to resolution of VOC (h) (composite end point)|| | |||
| N | 43 | 33 | |
| Mean (SD)* | 110.13 (128.04) | 147.72 (139.42) | |
| LS mean (SE)†|| | 103.64 (20.85) | 144.60 (23.54) | .192 |
| Median (CI)ঠ| 69.6 (44.3, 115.5) | 132.9 (67.0, 164.2) | .187 |
| Time to sustained decrease in VAS score (h) | |||
| N | 29 | 23 | |
| Mean (SD)* | 55.05 (51.03) | 77.43 (77.39) | |
| LS mean (SE)†¶ | 54.13 (12.21) | 75.65 (13.68) | .240 |
| Median (CI)ঠ| 72.0 (34.5, 115.7) | 125.3 (19.7, 180.2) | .428 |
| Time to transition to oral analgesia (h) | |||
| N | 43 | 33 | |
| Mean (SD)* | 110.35 (129.58) | 157.69 (134.71) | |
| LS mean (SE)†¶ | 108.58 (20.78) | 155.55 (23.77) | .137 |
| Median (CI)ঠ| 71.7 (48, 116.1) | 147.4 (60.9, 181.0) | .089 |
| Time to sustained decrease in VAS score and transition to oral analgesia (h) | |||
| N | 29 | 23 | |
| Mean (SD)* | 92.78 (76.07) | 137.85 (90.73) | |
| LS mean (SE)†¶ | 87.77 (15.86) | 135.17 (17.47) | .047 |
| Median (CI)ঠ| 128.0 (57.7, 156.9) | 181.0 (97.7, 217.0) | .197 |
| Time to agreement about readiness for discharge (h) | |||
| N | 38 | 28 | |
| Mean (SD)* | 100.50 (80.14) | 136.73 (122.30) | |
| LS mean (SE)†¶ | 97.63 (16.58) | 133.12 (19.46) | .165 |
| Median (CI)ঠ| 72.5 (60.9, 139.1) | 137.4 (83.2, 165.7) | .151 |
| Time to discharge (h) | |||
| N | 43 | 33 | |
| Mean (SD)* | 124.92 (129.60) | 175.27 (149.59) | |
| LS mean (SE)†¶ | 118.79 (21.73) | 173.53 (24.54) | .096 |
| Median (CI)ঠ| 72.2 (59.9, 121.0) | 156.1 (75.4, 185.8) | .092 |
| Resolution of VOC achieved at various time points cumulative, N (%) | |||
| N | 43 | 33 | |
| 48 h | 17 (39.5) | 8 (24.2) | .219 |
| 72 h | 22 (51.2) | 11 (33.3) | .162 |
| 96 h | 25 (58.1) | 13 (39.4) | .165 |
| 120 h | 28 (65.1) | 15 (45.5) | .106 |
| Hospital length of stay (h) | |||
| N | 43 | 33 | |
| Mean (SD)* | 132.36 (129.80) | 183.20 (148.26) | |
| LS mean (SE)† | 131.65 (21.50) | 182.07 (24.64) | .124 |
| Median (CI)‡ | 84.8 (66.1, 132.4) | 165.1 (79.6, 187.8) | .093 |
| VAS at discharge (cm) | |||
| N | 43 | 33 | |
| Mean (SD)* | 3.02 (2.75) | 4.03 (2.97) | |
| Median (range)* | 2.8 (0, 9.8) | 3.6 (0, 10) | |
| Cumulative parenteral opioid use (mg/kg MEU) | |||
| Mean (SD)* | 12.92 (20.8) | 57.91 (109.8) | |
| LS mean (SE)†¶ | 9.62 (11.55) | 55.59 (13.07) | .010 |
| Median (range)* | 3.42 (0.1, 93.5) | 11.17 (0, 487.3) |
| . | GMI-1070 . | Placebo . | P . |
|---|---|---|---|
| First medical evaluation to treatment (h) | |||
| N | 43 | 33 | |
| Mean (SD)* | 14.85 (4.36) | 15.35 (5.33) | |
| LS mean (SE)† | 14.54 (0.72) | 15.37 (0.82) | .445 |
| Median (CI)‡ | 14.7 (13.1, 16.6) | 15.2 (12.5, 17.3) | .457 |
| Reason for discontinuation of study drug, N (%) | |||
| N | 43 | 33 | .288§ |
| Resolution of VOC | 28 (65.1) | 19 (57.6) | |
| Received maximum (15 doses) | 6 (14) | 5 (15.2) | |
| No improvement on day 5 | 1 (2.3) | 2 (6.1) | |
| AE | 8 (18.6) | 4 (12.1) | |
| Other | 0 (0) | 3 (9.1) | |
| End point component first achieved, N (%) | |||
| N | 43 | 33 | |
| Sustained decrease in VAS score and transition to oral analgesia | 19 (44.2) | 15 (45.5) | |
| Agreement about readiness for discharge | 18 (41.9) | 16 (48.5) | |
| Hospital discharge | 8 (18.6) | 4 (12.1) | |
| Time to resolution of VOC (h) (composite end point)|| | |||
| N | 43 | 33 | |
| Mean (SD)* | 110.13 (128.04) | 147.72 (139.42) | |
| LS mean (SE)†|| | 103.64 (20.85) | 144.60 (23.54) | .192 |
| Median (CI)ঠ| 69.6 (44.3, 115.5) | 132.9 (67.0, 164.2) | .187 |
| Time to sustained decrease in VAS score (h) | |||
| N | 29 | 23 | |
| Mean (SD)* | 55.05 (51.03) | 77.43 (77.39) | |
| LS mean (SE)†¶ | 54.13 (12.21) | 75.65 (13.68) | .240 |
| Median (CI)ঠ| 72.0 (34.5, 115.7) | 125.3 (19.7, 180.2) | .428 |
| Time to transition to oral analgesia (h) | |||
| N | 43 | 33 | |
| Mean (SD)* | 110.35 (129.58) | 157.69 (134.71) | |
| LS mean (SE)†¶ | 108.58 (20.78) | 155.55 (23.77) | .137 |
| Median (CI)ঠ| 71.7 (48, 116.1) | 147.4 (60.9, 181.0) | .089 |
| Time to sustained decrease in VAS score and transition to oral analgesia (h) | |||
| N | 29 | 23 | |
| Mean (SD)* | 92.78 (76.07) | 137.85 (90.73) | |
| LS mean (SE)†¶ | 87.77 (15.86) | 135.17 (17.47) | .047 |
| Median (CI)ঠ| 128.0 (57.7, 156.9) | 181.0 (97.7, 217.0) | .197 |
| Time to agreement about readiness for discharge (h) | |||
| N | 38 | 28 | |
| Mean (SD)* | 100.50 (80.14) | 136.73 (122.30) | |
| LS mean (SE)†¶ | 97.63 (16.58) | 133.12 (19.46) | .165 |
| Median (CI)ঠ| 72.5 (60.9, 139.1) | 137.4 (83.2, 165.7) | .151 |
| Time to discharge (h) | |||
| N | 43 | 33 | |
| Mean (SD)* | 124.92 (129.60) | 175.27 (149.59) | |
| LS mean (SE)†¶ | 118.79 (21.73) | 173.53 (24.54) | .096 |
| Median (CI)ঠ| 72.2 (59.9, 121.0) | 156.1 (75.4, 185.8) | .092 |
| Resolution of VOC achieved at various time points cumulative, N (%) | |||
| N | 43 | 33 | |
| 48 h | 17 (39.5) | 8 (24.2) | .219 |
| 72 h | 22 (51.2) | 11 (33.3) | .162 |
| 96 h | 25 (58.1) | 13 (39.4) | .165 |
| 120 h | 28 (65.1) | 15 (45.5) | .106 |
| Hospital length of stay (h) | |||
| N | 43 | 33 | |
| Mean (SD)* | 132.36 (129.80) | 183.20 (148.26) | |
| LS mean (SE)† | 131.65 (21.50) | 182.07 (24.64) | .124 |
| Median (CI)‡ | 84.8 (66.1, 132.4) | 165.1 (79.6, 187.8) | .093 |
| VAS at discharge (cm) | |||
| N | 43 | 33 | |
| Mean (SD)* | 3.02 (2.75) | 4.03 (2.97) | |
| Median (range)* | 2.8 (0, 9.8) | 3.6 (0, 10) | |
| Cumulative parenteral opioid use (mg/kg MEU) | |||
| Mean (SD)* | 12.92 (20.8) | 57.91 (109.8) | |
| LS mean (SE)†¶ | 9.62 (11.55) | 55.59 (13.07) | .010 |
| Median (range)* | 3.42 (0.1, 93.5) | 11.17 (0, 487.3) |
Descriptive mean or median.
LS mean obtained by ANCOVA.
Median obtained by Kaplan-Meier estimate.
P value for overall association between reason for discontinuation and treatment group.
Primary end point.
Secondary end points.
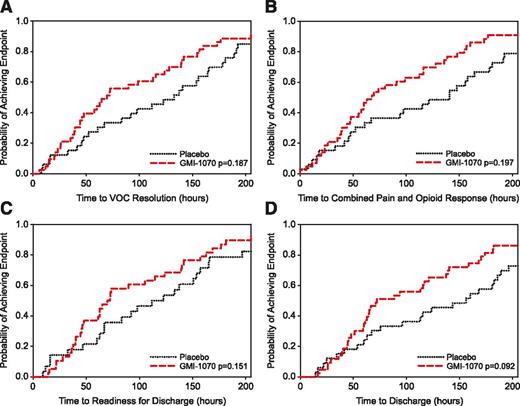
Time to resolution of VOC
All subjects reached the primary efficacy end point of resolution of VOC. The time to resolution of VOC was substantially, although not statistically significantly, shorter (28% and 48% reduction in mean and median time to resolution) for subjects receiving GMI-1070 than for those receiving placebo (P values from ANCOVA model and log-rank test = .192 and .187, respectively) (Figure 2A; Table 2). GMI-1070 resulted in absolute decreases in time to resolution of VOC of 41 hours (mean) and 63 hours (median). In addition, proportionally more subjects receiving GMI-1070 than placebo achieved resolution of VOC at 48, 72, 96, and 120 hours (Table 2). The criteria for resolution of VOC reached first among all subjects were most often a sustained decrease in VAS score, accompanied by transition to oral analgesics (34 subjects) and documentation of patient-physician agreement about readiness for discharge (34 subjects) (Table 2).
Time to resolution of VOC (primary end point) and component (secondary) end points. (A) Kaplan-Meier plot of time to resolution of VOC, defined as composite primary end point (see “Methods”). Maximum times were 758.1 hours (GMI-1070) and 622.8 hours (placebo). (All subjects achieved this end point; N = 76). (B) Kaplan-Meier plot of time to first ≥ 1.5 cm sustained decrease in VAS pain score and transition to oral analgesia for those achieving this component at any time (N = 52). Maximum times were 307.7 hours (GMI-1070, N = 29) and 281.5 hours (placebo, N = 23). (C) Kaplan-Meier plot of time to documented readiness for discharge for those achieving this component at any time (N = 66). Maximum times were 336.7 hours (GMI-1070, N = 38) and 588.7 hours (placebo, N = 28). (D) Kaplan-Meier plot of time to order for discharge. Maximum times were 758.1 hours (GMI-1070) and 622.8 hours (placebo). (All subjects achieved this end point; N = 76).
Time to resolution of VOC (primary end point) and component (secondary) end points. (A) Kaplan-Meier plot of time to resolution of VOC, defined as composite primary end point (see “Methods”). Maximum times were 758.1 hours (GMI-1070) and 622.8 hours (placebo). (All subjects achieved this end point; N = 76). (B) Kaplan-Meier plot of time to first ≥ 1.5 cm sustained decrease in VAS pain score and transition to oral analgesia for those achieving this component at any time (N = 52). Maximum times were 307.7 hours (GMI-1070, N = 29) and 281.5 hours (placebo, N = 23). (C) Kaplan-Meier plot of time to documented readiness for discharge for those achieving this component at any time (N = 66). Maximum times were 336.7 hours (GMI-1070, N = 38) and 588.7 hours (placebo, N = 28). (D) Kaplan-Meier plot of time to order for discharge. Maximum times were 758.1 hours (GMI-1070) and 622.8 hours (placebo). (All subjects achieved this end point; N = 76).
The time to transition to oral analgesics was also decreased in the GMI-1070 group compared with placebo; this reduction was 76 hours in the pooled GMI-1070 group (log rank P = .089) and reached statistical significance in the pediatric GMI-1070 population (87.8 hours, log rank P = .037). Although 24 subjects (31.6%) did not achieve a sustained reduction in VAS while hospitalized, active treatment was nevertheless associated with a 21-hour and 53-hour (mean and median) decrease (P = .24 and .43, respectively) in time to first sustained reduction in VAS among all subjects meeting that end point. Similarly, median time to reach the combined end point of sustained reduction in VAS score and transition to oral analgesics was substantially reduced for the GMI-1070 group vs placebo, although not statistically significantly so (Figure 2B). Both time to agreement about readiness for discharge and time to discharge were markedly but not statistically significantly shortened with GMI-1070 (Figure 2C-D).
Safety
AEs, including common complications of VOC, occurred at similar rates in both groups (Table 3). SAEs occurred in 30.3% of subjects in each group, with rehospitalization for VOC within 30 days from discharge being the most common (Table 3); no deaths occurred during the study. Although subjects experienced leukocytosis in the phase 1 study of GMI-1070,21 this was not observed in the present study. Furthermore, pediatric subjects experienced a similar SAE rate (30.8% and 43% in the treatment and placebo groups, respectively), as did adults. ACS occurred in 9 of 76 subjects (11.8%) without a significant difference between GMI-1070 and placebo (Table 3), although the rate of ACS was higher in pediatric subjects (20%) than in adults (9%). Because the drug is renally cleared, creatinine levels were closely monitored but did not increase with GMI-1070 use. Acute generalized exanthematous pustulosis occurred in 1 adult after completion of GMI-1070 therapy and patient discharge; it resolved without complications. Other types of rashes were mild and occurred in both groups.
Frequency of SCD-related events and treatment-emergent AEs
| . | Treatment group . | |
|---|---|---|
| . | GMI-1070 (N = 43) . | Placebo (N = 33) . |
| SCD-related events | ||
| ACS | 6 (14.0) | 3 (9.1) |
| RBC transfusion | 15 (34.9) | 17 (51.5) |
| ICU stay | 0 | 1 (3) |
| Death | 0 | 0 |
| Readmission for VOC (14 d) | 4 (9.3) | 3 (9.1) |
| Readmission for VOC (30 d) | 9 (20.9) | 7 (21.2) |
| Treatment-emergent AEs | ||
| Gastrointestinal disorders | 18 (41.9) | 12 (36.4) |
| Rash | 6 (14.0) | 2 (6.1) |
| Hepatobiliary disorders | 2 (4.7) | 2 (6.1) |
| Renal/urinary disorders | 3 (7.0) | 2 (6.1) |
| Pyrexia | 8 (18.6) | 6 (18.2) |
| Headache | 8 (18.6) | 4 (12.1) |
| . | Treatment group . | |
|---|---|---|
| . | GMI-1070 (N = 43) . | Placebo (N = 33) . |
| SCD-related events | ||
| ACS | 6 (14.0) | 3 (9.1) |
| RBC transfusion | 15 (34.9) | 17 (51.5) |
| ICU stay | 0 | 1 (3) |
| Death | 0 | 0 |
| Readmission for VOC (14 d) | 4 (9.3) | 3 (9.1) |
| Readmission for VOC (30 d) | 9 (20.9) | 7 (21.2) |
| Treatment-emergent AEs | ||
| Gastrointestinal disorders | 18 (41.9) | 12 (36.4) |
| Rash | 6 (14.0) | 2 (6.1) |
| Hepatobiliary disorders | 2 (4.7) | 2 (6.1) |
| Renal/urinary disorders | 3 (7.0) | 2 (6.1) |
| Pyrexia | 8 (18.6) | 6 (18.2) |
| Headache | 8 (18.6) | 4 (12.1) |
Values are N (%). No differences between GMI-1070 and placebo groups were statistically significant.
Secondary efficacy end points and subgroup analyses
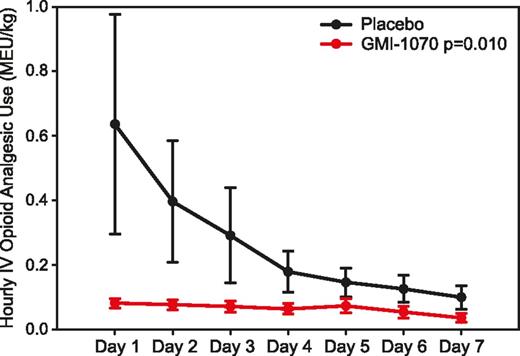
We performed statistical analyses of a number of secondary end points and subgroups, in order to better understand the outcomes of this study. Overall, an 83% reduction in mean cumulative IV opioid analgesic use (mg/kg MEU) was seen in the active treatment group (LS means: 9.62 and 55.59 in active and placebo groups, respectively; P = .010). With a nonparametric approach, a 69% reduction was seen for GMI-1070 (medians were 3.42 and 11.17 mg/kg MEU, respectively; P = .056 by Wilcoxon rank-sum test). The effect of GMI-1070 on opioid use was seen within 24 hours (Figure 3).
Mean hourly opioid use by day. Total parenteral opioid use per each 24-hour interval was converted to MEU and calculated as a mg/kg dose, and then the mean of each time interval was graphed by treatment group. All subjects were included for each time point; values of 0 were entered for subjects not receiving IV opioids or who had been discharged.
Mean hourly opioid use by day. Total parenteral opioid use per each 24-hour interval was converted to MEU and calculated as a mg/kg dose, and then the mean of each time interval was graphed by treatment group. All subjects were included for each time point; values of 0 were entered for subjects not receiving IV opioids or who had been discharged.
Pain scores, however, were not informative. Mean (SD) VAS scores at presentation were 8.28 (1.57) and 8.97 (1.52) for active and placebo groups, respectively, and ranged from 5 to 10 in both. Mean (SD) VAS scores at discharge were 3.02 (2.75) and 4.03 (2.97) for active and placebo groups, respectively, and ranged from 0 to ∼10 in both groups.
When analyzed separately, pediatric and adult subjects receiving GMI-1070 showed similar reductions in time to resolution of VOC, including time to discharge (data not shown), compared with the placebo group. Excluding the 3 subjects inadvertently enrolled with hemoglobin SC (HbSC) from analyses did not substantially change the results or conclusions drawn. Time to resolution of VOC for subjects receiving GMI-1070 or placebo was also not affected by HU use.
Both the low and high doses of GMI-1070 were associated with improvement in time to resolution of VOC (supplemental Table 1), although these differences were not statistically significant. In addition, the baseline LOS, and therefore time to resolution of VOC, changed over the course of the study. This was evident in the change in placebo group values for the 2 dose cohorts; nevertheless, both cohorts showed similar responses when compared with their concurrent placebo controls. For the subjects enrolled after the drug dose was doubled, mean time to resolution of VOC increased for both the active and placebo groups. In the placebo group, time to resolution of VOC before change in dose was 113 hours (LS mean) and 98 hours (median), whereas values of 166 hours (LS mean) and 146 hours (median) were observed after the change in dose (supplemental Table 1). However, frequency of prior VOC (>3 hospitalizations in the past year) was assessed in the ANCOVA model for effect on efficacy outcomes for GMI-1070 and was found not to impact the results. In addition, both dose cohorts showed similar differences in their cumulative IV opioid use during hospitalization (supplemental Table 1).
In addition, across all subgroups assessed, proportionally more subjects receiving active treatment achieved resolution of VOC at 48, 72, 96, and 120 hours (supplemental Figure 2), reaching statistical significance in the low-dose cohort at 120 hours (P = .039) (supplemental Table 1). The same directional change was observed for the pooled active group (P = .106 at 120 hours) and trended toward significance for the pediatric population at the low dose (P = .07 at 120 hours). Remarkably, in the high-dose cohort, 48% of subjects treated with GMI-1070 achieved resolution of VOC by 72 hours, whereas only 25% of the concurrent placebo group achieved resolution of VOC by that time point (P = .135).
Pharmacokinetics
The target GMI-1070 plasma concentration for this phase 2 study was based on levels resulting in improved blood flow, decreased cell adhesion, and improved survival in a SCD mouse model of VOC.18 Human PK data in both healthy volunteers and in SCD patients in steady state were used to estimate the dose regimen required to reach that plasma concentration (>20 μg/mL).21 However, the interim population PK analysis showed that the desired GMI-1070 plasma level was achieved for only 60% of the dosing interval; no covariates examined explained this difference. A doubling of both the initial loading and maintenance doses was calculated to achieve a minimum concentration (Cmin) of 20 μg/mL for 86% of the dosing interval. Therefore, the dose was doubled for the remainder of the subjects studied (n = 45, including 10 pediatric subjects), resulting in plasma levels above the targeted threshold in subsequent PK analyses. GMI-1070 clearance was entirely renal. Moreover, body size and age did not influence GMI-1070 clearance, despite the large range of body weights (32 to 109 kg) and ages (12 to 51 years) in this study.
Discussion
This phase 2 clinical trial, although conducted primarily to establish the safety of a novel treatment in the intended patient population, was also designed to provide supportive efficacy data for a possible pivotal phase 3 trial in a rare disease indication. Clinical trials in rare diseases face the extraordinary challenge of evaluating efficacy in a limited patient population, and thus require efficient design and use of enrollment. We believe this study has accomplished both goals by utilizing a randomized placebo-controlled adaptive trial design that evaluated both safety and a variety of efficacy end points to inform phase 3 study design.
Use of GMI-1070 early in the hospital course of VOC nominally improved multiple outcomes, including time to resolution of VOC, LOS, and requirement for parenteral opioid analgesia, without increased AEs. Although observed differences rarely reached statistical significance, the improvements seen in time to resolution of VOC and LOS would be clinically meaningful and important if verified. Most remarkably, improvements, some quite large, were seen in every efficacy end point explored, whereas previous trials in VOC have shown only modest, if any, improvements in some but not all end points measured.22-24 In addition, the improvements observed here appeared independent of age and HU use and were achieved without worsening of other parameters, such as requirement for blood transfusion or frequency of AEs. Analyses of outcomes excluding subjects inadvertently included with HbSC revealed no differences in effects or safety.
Although this randomized phase 2 study was powered to detect an efficacy signal and effect size estimation for planning a phase 3 study, patient variability, characteristic of SCD, may have been accentuated by the adaptive study design and precluded achieving significance for the primary end point in this sample size. Institutional variability in medical management did not appear to contribute to overall patient variability, as a subgroup analysis of the highest enrolling sites revealed that variability in patient outcomes was already present at the site level. Limitations of the study included the broad range of values in efficacy outcomes and increased patient variability toward the latter part of the study, as well as slight differences in baseline characteristics, which could have affected the observed differences between treatment groups. Analysis methods were adjusted for certain baseline characteristics and other variables in outcomes to the extent possible.
Changes in inclusion and exclusion criteria during the study may have resulted in inclusion of patients with more severe VOC as the study progressed. After the study drug dose was doubled, mean time to resolution of VOC increased for both the active drug and placebo groups, as did total IV opioid use. Principal investigator comfort with first-time use of GMI-1070 in VOC may have impacted patient selection for participation, especially early in the course of the study. Other unidentified factors may also have contributed. Nevertheless, although the GMI-1070 and placebo groups in the later cohort showed a sizable increase in time to resolution of VOC, LOS, and total IV opioid use compared with subjects in the early phase of the study, comparisons consistently showed improvement in the GMI-1070 groups, even though the differences were not statistically significant.
VAS scores were the least helpful end point examined in this study, as they were highly variable among subjects at study entry, and a significant proportion of subjects never reached the criteria for sustained VAS score reduction prior to discharge. VAS scores at discharge ranged from 0 to 10, with large SDs (2.8 and 3.0 for GMI-1070 and placebo, respectively). Despite this, subjects who received GMI-1070 had lower mean daily VAS scores than those receiving placebo during up to 7 days of hospitalization.
As expected, ACS occurred as a complication of VOC in both treatment groups, at a rate of 11.8% overall, and not significantly different in the treatment and control groups. In the recent PROACTIVE study, ACS occurred in 22 (9.4%) of 233 patients aged 2 to 68 years hospitalized for pain.25 About half of all ACS cases first present as VOC, after which ACS typically becomes evident 2.5 days after admission.26 In our study, most cases of ACS occurred early. In the GMI-1070 group, 4 of 6 cases occurred before 48 hours of treatment; in the placebo group, 1 of 3 cases occurred before 48 hours. This pattern supports the likelihood that GMI-1070 treatment neither increased nor decreased the rate of development of ACS.
We also examined the rate of rehospitalization for VOC. Rehospitalization within 30 days after discharge for VOC is frequent in SCD and was the most common SAE observed in our study, occurring in 20.9% and 21.2% of subjects receiving GMI-1070 and placebo, respectively. Previous studies have documented 30-day readmission rates ranging from 28% to 50%27-29 and 1- and 2-week readmission rates as high as 16% and 28%.27,28 Thus, the readmission rate in our trial was substantial but lower than those previously documented. Although >30% of subjects had had ≥ 3 hospitalizations in the 12 months preceding study entry, a known risk factor for readmission,30 the mandated follow-up study visits at 7 ± 3 and 28 ± 5 days ensured close out-patient follow up. Possibly, this careful follow up reduced the frequency of but by no means prevented rehospitalization. Surprisingly, although children had a lower rate of previous admissions, their readmission rate (20%) in the study was similar to that of adults (21%). The treatment arm had no significant effect on readmission rate in either age group.
Unexpected findings in this study included the marked effect on hourly opioid use during the first 24 hours of GMI-1070 treatment. Combined with differences in VAS scores and time to VOC resolution with GMI-1070, this observation supports the likelihood that GMI-1070 effectively interferes with the pathophysiology of progression of VOC and may promote its early reversal. Biomarker data are being analyzed to discern possible correlation of mechanism-associated changes with clinical outcomes and will be reported separately. Another unexpected finding was the lack of leukocytosis with GMI-1070 treatment, whereas earlier studies in steady-state SCD patients documented frequent leukocytosis in response to GMI-1070, presumably due to demargination. We theorize that, during VOC, demargination has already occurred (in response to inflammation). In addition, the rate of pyrexia was the same in both groups and no serious infections occurred. It was unknown before this clinical trial if inhibiting selectin-mediated leukocyte trafficking would impact infection rates in functionally asplenic individuals with SCD VOC; the complete absence of any serious bacterial infection suggests that targeted selectin inhibition may be reasonable in this population. Finally, we found, somewhat unexpectedly, that renal clearance of GMI-1070 was faster in patients experiencing VOC than that previously reported in steady state,31 possibly due to vigorous hydration, increased metabolic state, or increased cardiac output. Moreover, body size and age did not influence GMI-1070 clearance, suggesting that, over the range of ages, body sizes, and renal function in the present study, a fixed rather than weight-based dose may be appropriate.
Three relatively large trials of novel therapies for VOC have been completed in the last 15 years. The trials of purified poloxamer 188 and inhaled nitric oxide, respectively, showed only minimal and no improvement in time to resolution of VOC between treatment groups.22,23 More recently, a study of tinzaparin vs placebo during VOC was reported to improve time to resolution of VOC,24 although both treatment of pain and definition of resolution of VOC were distinctly different from those typically used in the United States. Interestingly however, tinzaparin may, like other heparinoids, have an anti-selectin effect that could account for its possible efficacy independently of its effect on coagulation.
We report herein the initial evaluation of GMI-1070 (rivipansel), a pan-selectin antagonist, for the treatment of VOC in SCD. Large, but mostly not statistically significant improvements were observed in multiple outcomes, including time to resolution of VOC, time to hospital discharge, and amount of parenteral opioid analgesia required. These effects were observed in all subset analyses, such as adults vs children and subjects with and without HU. Against the background of minimal progress to date in treating VOC, our results support moving forward with a phase 3 study of GMI-1070 (#NCT02187003, www.clinicaltrials.gov) in order to confirm the efficacy signal in VOC. Ultimately, we expect that treatment of VOC may involve multimodality therapy targeting different contributing pathophysiologic processes, including cell adhesion, activation of inflammatory and pain pathways, and other pathophysiologic processes.32
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the hard work and thoughtful advice of all site investigators and research coordinators (O. Alvarez and T. Hustace, University of Miami Miller School of Medicine; J. Jonassaint, Duke University Medical Center; C. Driscoll and C. Bell, Children’s Hospital at Montefiore; V. Gordeuk, L. Krauz, and M. Girotti, University of Illinois at Chicago; W. Hagar, M. Macarewich, and S. Murphy, Alta Bates Summit Medical Center/Children’s Hospital at Oakland; K. Hassell and J. McAfee, University of Colorado Denver Health Sciences Center; J. Handy, Children’s National Medical Center; M. Byrne, K. Stiegler, and D. Ross, Children’s Hospital of Pittsburgh [UPMC]; A. Kutlar, L. Wells, L. Bowman, and N. Barrett, Georgia Health Sciences University; C. Williams, Johns Hopkins School of Medicine; M. Henson, University of Texas Southwestern Medical Center; I. Odame and M. Merelles-Pulcini, The Hospital for Sick Children, Toronto; C. Quinn and K. Thueneman, Cincinnati Children’s Hospital Medical Center, M. G. Smith and G. Thomas, University of Mississippi Medical Center; M. Madu, Virginia Commonwealth University Medical Center; K. Smith-Whitley and H. Enninful-Eghan, The Children’s Hospital of Philadelphia; and M. Garcia and D. Tsai, University of CA Davis Medical Center); the staff who supported this study (GMI: Lauren Berning, Henry Flanner, Martina Hemmer, Christine Kolata Nietubicz, and Shanti Rodriguez; Consultants to GMI: Kristen Hahn, LaTonya Hendricks, Maria Lempicki, Rebecca Seufert, and Carmen Wilberg, and INC: Mark Crisanti); and the members of the Data Safety and Monitoring Board (Carlton Dampier, Emory University, Karen Kesler, Rho Inc., Ellis Neufeld, Harvard University, and George Atweh, University of Cincinnati).
This study was funded and supported by GlycoMimetics, Inc. (Gaithersburg, MD) and contracted INC Research (Raleigh, NC) and Rho Inc. (Chapel Hill, NC) for data management and statistical analysis, respectively.
Authorship
Contribution: The study was designed by H.T. with close collaboration with M.J.T., other authors, and other clinicians in the field; M.J.T., T.W., T.L.M., L.M.D.C., L.K., S.L., L.L.H., and W.R.S. were responsible for acquisition of data; M.J.T., T.W., T.L.M., L.M.D.C., L.K., S.L., L.L.H., W.R.S., S.R., J.L.M., and H.T. were responsible for analysis and interpretation of data; M.J.T., T.W., and H.T. drafted the manuscript; M.J.T., T.W., T.L.M., L.M.D.C., L.K., S.L., L.L.H., W.R.S., S.R., J.L.M., and H.T. were responsible for critical revision of the manuscript for important intellectual content; S.R. and H.T. were responsible for statistical analysis; J.L.M. and H.T. provided administrative, technical, and material support; M.J.T. and H.T. supervised the study; and M.J.T., T.W., T.L.M., L.M.D.C., L.K., S.L., L.L.H., W.R.S., S.R., J.L.M., and H.T. performed research. M.J.T., T.W, and H.T. worked with Rho Inc. (Chapel Hill, NC) for statistical analyses; M.J.T. had full access to all of the data in the study and assumes responsibility for the integrity of the data and the accuracy of the data analyses; T.W. and H.T. contributed equally to this work; and all authors had access to the primary data of the study, reviewed, and approved the manuscript.
Conflict-of-interest disclosure: M.J.T., T.W., T.L.M., L.M.D.C., L.K., S.L., L.L.H., and W.R.S. received support for travel to study-related meetings, and their institutions all received research support through clinical trial agreements. S.R. is an employee of Rho Inc. J.L.M. and H.T. are employed by and have equity ownership in GlycoMimetics, Inc. The following authors report consultancy income relevant to this work: M.J.T. (Biogen Idec, Mast Pharmaceuticals, and Pfizer Inc.); T.W. (Pfizer Inc.); T.L.M. (Pfizer Inc.); L.M.D.C. (Pfizer Inc.); L.K. (Pfizer Inc.); L.L.H. (Gerson Lehrman Group); and W.R.S. (Pfizer Inc.). The following authors report other research funding: M.J.T. (National Institutes of Health [NIH], Dilafor AB, and Doris Duke Charitable Foundation); T.W. (NIH, Selexys Pharmaceutical Corp., Emmaus, Inc., and Eli Lilly, Inc.); L.M.D.C. (NKT Therapeutics and Novella Clinical); and L.L.H. (NIH, Health Resources and Services Administration, Doris Duke Charitable Foundation, Mast Therapeutics, Purdue Pharma, Selexys, and Emmaus).
Correspondence: Marilyn J. Telen, Box 2615, Room 333, Duke University Medical Center, Durham, NC 27710; e-mail: marilyn.telen@duke.edu.