Key Points
Mice deficient in HRG have normal hemostasis, but demonstrate accelerated thrombosis via the contact system.
HRG abrogates nucleic acid–driven coagulation and serves as a novel modulator of the contact system in vivo.
Abstract
Factor (F) XII, a key component of the contact system, triggers clotting via the intrinsic pathway, and is implicated in propagating thrombosis. Although nucleic acids are potent activators, it is unclear how the contact system is regulated to prevent uncontrolled clotting. Previously, we showed that histidine-rich glycoprotein (HRG) binds FXIIa and attenuates its capacity to trigger coagulation. To investigate the role of HRG as a regulator of the intrinsic pathway, we compared RNA- and DNA-induced thrombin generation in plasma from HRG-deficient and wild-type mice. Thrombin generation was enhanced in plasma from HRG-deficient mice, and accelerated clotting was restored to normal with HRG reconstitution. Although blood loss after tail tip amputation was similar in HRG-deficient and wild-type mice, carotid artery occlusion after FeCl3 injury was accelerated in HRG-deficient mice, and HRG administration abrogated this effect. To confirm that HRG modulates the contact system, we used DNase, RNase, and antisense oligonucleotides to characterize the FeCl3 model. Whereas DNase or FVII knockdown had no effect, carotid occlusion was abrogated with RNase or FXII knockdown, confirming that FeCl3-induced thrombosis is triggered by RNA in a FXII-dependent fashion. Therefore, in a nucleic acid–driven model, HRG inhibits thrombosis by modulating the intrinsic pathway of coagulation.
Introduction
Blood coagulation is initiated via 2 distinct pathways: (1) the extrinsic pathway, which is initiated by the tissue factor (TF)/factor (F) VIIa complex; and (2) the intrinsic pathway, which is initiated by FXII activation.1 Studies in knockout mice identify the TF/FVIIa complex as an important driver of hemostasis and modulator of thrombosis.2-4 In contrast, the contact system within the intrinsic pathway plays little or no part in hemostasis. Thus, patients with congenital deficiency of FXII or its cofactors, prekallikrein (PK) and high-molecular-weight kininogen (HK), have no bleeding diathesis, and most patients with FXI deficiency only experience bleeding with surgery or trauma.5 However, there is mounting evidence that the contact system is important for thrombus stabilization and propagation.6 Thus, mice deficient in FXII or FXI exhibit attenuated thrombosis at sites of arterial or venous injury,7-9 and depletion of FXII or FXI with antisense oligonucleotides (ASOs), or inhibition of FXIIa or FXIa with antibodies or inhibitors, reduces thrombosis in animal models.10-14 With increasing evidence that they contribute to thrombosis, but have little role in hemostasis, FXII and FXI have emerged as attractive targets for new anticoagulants.6,15
The intrinsic pathway is initiated when FXII is activated on polyanionic surfaces, a process enhanced by PK and HK. FXIIa activates FXI, which propagates the procoagulant response and culminates in thrombin generation and fibrin formation.5 Recently, naturally occurring polyphosphates, such as DNA, RNA, and inorganic polyphosphate, were shown to activate the contact system. DNA and RNA are released from stimulated, apoptotic, or necrotic cells, whereas polyphosphate is released from activated platelets.16-18 In purified systems, DNA and RNA promote FXII activation, whereas platelet polyphosphate is less potent.19,20 Plasma levels of cell-free DNA are increased in murine models of ischemic stroke and venous thrombosis, and DNase treatment attenuates thrombosis in these models.21,22 Likewise, RNase, but not DNase, was reported to attenuate FeCl3-induced arterial thrombosis in mice, a finding that implicates RNA as a driver of clotting in this model.19,23 With potential physiologic activators of the contact system now identified, it is important to understand how the contact system is downregulated.
Histidine-rich glycoprotein (HRG) is an ∼75 kDa glycoprotein that circulates in plasma at 1.5 to 2.0 μM. A modular protein with distinct structural domains, HRG has been implicated in diverse processes, including coagulation, immunity, and angiogenesis.24,25 HRG binds multiple ligands, such as zinc, fibrin(ogen), heparin, plasmin(ogen), DNA, kallikrein, and FXIIa.26-28 Because of its numerous interactions, HRG is hypothesized to serve as an adaptor or accessory protein.26
Previously, we showed that the activated partial thromboplastin time (aPTT) is shortened when human plasma is immunodepleted of HRG, whereas the prothrombin time (PT) is unchanged,27 findings that localize the HRG effect to the intrinsic pathway. HRG binds FXIIa with high affinity and attenuates its capacity to activate FXI and propagate coagulation.27 In this study, we used HRG-deficient mice to test the hypothesis that HRG modulates thrombosis in vivo without affecting hemostasis.
Methods
Materials
HRG was isolated from human plasma by nickel-NTA chromatography.27 RecombiPlasTin and APTT-SP were obtained from Instrumentation Laboratory (Bedford, MA). Polyclonal goat anti-human FXII and anti-mouse FVII IgG (GAFXII-AP and AF3305, respectively) were obtained from Affinity Biologicals (Ancaster, ON) and R&D Systems (Abingdon, UK), respectively. A sheep polyclonal antibody against human HRG (Affinity Biologicals) was affinity-purified using an HRG-Sepharose column, as described in supplemental Methods available on the Blood Web site. Antibodies were conjugated to horseradish peroxidase using Lightning-Link (Innova Biosciences, Cambridge, UK). Mouse anti-human fibrin IgG (T2G1) and rat anti-mouse CD41 IgG (MWReg30) were obtained from Accurate Chemical and Scientific Co. (Westbury, NY) and BD Biosciences (Mississauga, ON, Canada), respectively. RNase A (DNase and protease-free) was obtained from Thermo Scientific (Waltham, MA) and was heat-inactivated for 15 minutes at 95°C. DNase I was kindly provided by Roche (Mississauga, ON, Canada). AlexaFluor 350 succinimidyl ester, Sytox RNA-select, goat anti-mouse AlexaFluor 647, goat anti-rat AlexaFluor 647, and goat anti-rat AlexaFluor 488 were obtained from Life Technologies (Burlington, ON, Canada). Phosphatidylcholine-phosphatidylserine (3:1) vesicles (PCPS) were prepared as described.29
Nucleic acid preparations.
RNA and DNA were isolated from cultured A549 non–small cell lung cancer cells, a generous gift from Dr. P. Liaw, using RNeasy Plus mini and DNeasy mini isolation kits, respectively (Qiagen, Mississauga, ON, Canada), according to the manufacturer’s protocol. RNA and DNA were quantified by measuring absorbance at 260 nm with a BioPhotometer Plus spectrophotometer (Eppendorf, Mississauga, ON, Canada).
Mice.
HRG−/− mice were maintained on a pure C57BL/6 background after more than 10 generations of backcrossing.30 Wild-type C57BL/6 mice (Charles River Laboratories) were crossed with HRG−/− mice to generate HRG+/− mice. Polymerase chain reaction analysis was used for genotyping as described.31 Mice were housed in microisolator cages exposed to constant light-dark cycles and had free access to food and water. Murine plasma was prepared and HRG levels were quantified by immunoblot analysis as described in supplemental Methods. Weight- and age-matched female mice were used for all experiments. Animal utilization protocols were approved by the Animal Research Ethics Board at McMaster University, and studies were performed in accordance with Canadian Council on Animal Care guidelines.
Antisense oligonucleotides (ASOs).
Control and mouse-specific FXII- and FVII-directed ASOs were synthesized by Isis Pharmaceuticals (Carlsbad, CA) and prepared as described previously.11 Wild-type and HRG−/− mice were given 20 mg/kg FXII, FVII, or control ASO via twice-weekly subcutaneous injections for up to 6 weeks. The effect of the ASOs on protein expression was assessed by immunoblot analysis, as described in Supplemental methods.
Plasma clotting assays.
Dilute clotting assays were performed as described,27 with some modifications. The intrinsic pathway was assessed using aPTT reagent diluted 1:5 in 125 mM imidazole buffer, pH 7.0, whereas the extrinsic pathway was evaluated using RecombiPlasTin diluted 1:200 in 20 mM Tris-HCl, pH 7.4, 150 mM NaCl (Tris-buffered saline [TBS]). Fifty μL of dilute aPTT reagent or RecombiPlasTin was added to wells of a 96-well plate containing 50 μL of citrated plasma from HRG−/−, HRG+/−, or wild-type mice supplemented with 30 μM PCPS. The effect of HRG on nucleic acid–induced activation of the intrinsic pathway was examined by incubating 25 μL of a 30-µg/mL RNA or DNA solution diluted in imidazole buffer with 50 µL of murine plasma supplemented with 60 μM PCPS in the absence or presence of 25 μL human HRG at concentrations up to 3 µM. Clotting was initiated by the addition of 50 μL of CaCl2 to final concentrations of 8.3 mM and 7.5 mM for the dilute aPTT and prothrombin time (PT), respectively. For plasma from mice treated with ASOs, the dilute aPTT was performed as described before, except the volume of plasma was reduced to 30 μL and clotting was initiated by the addition of 30 μL of CaCl2 to a final concentration of 8.3 mM. The dilute PT in plasma from ASO-treated mice was performed by adding 30 μL of plasma and 30 μL of a 1:400 dilution of RecombiPlasTin and CaCl2 to a final concentration of 12.5 mM. Absorbance was monitored at 405 nm at 37°C using a SpectroMax Plus plate reader (Molecular Devices, Sunnyvale, CA), and clot time was taken as the time to half-maximal absorbance.
Thrombin generation.
Thrombin generation was quantified by calibrated automated thrombography,32 with some modifications. To assess the intrinsic pathway, 16 μg/mL RNA or 20 μg/mL DNA prepared in imidazole buffer, or 10 μL of aPTT reagent at 1:2 dilution was added to wells of a black 96-well Costar plate (Lowell, MA) containing 40 μL of plasma from wild-type, HRG+/−, or HRG−/− mice supplemented with 30 µM PCPS. The extrinsic pathway was evaluated by adding 10 μL of RecombiPlasTin diluted 1:1000 in TBS and 50 µg/mL corn trypsin inhibitor (Enzyme Research Laboratories) to inhibit contact activation. Thrombin generation was initiated by adding 50 µL of solution containing 15 mM CaCl2 and 1 mM Z-Gly-Gly-Arg-AMC (Bachem, Torrance, CA), and substrate hydrolysis was monitored using a SpectraMax M5e plate reader (Molecular Devices). Thrombin-generation profiles were analyzed using Technothrombin TGA software (Technoclone, Vienna, Austria).
FeCl3-induced arterial thrombosis.
Wild-type, HRG+/−, and HRG−/− mice and HRG−/− mice supplemented with human HRG or saline were anesthetized with 2% to 5% isoflurane (Baxter, Deerfield, IL) delivered by facemask. The right common carotid artery was isolated and injury was induced by applying a 1 × 1-mm filter paper presoaked in 7.5% FeCl3 to the external surface for 1 minute; mild conditions were chosen to not induce thrombosis in wild-type mice for at least 30 minutes after application. Where indicated, the left jugular vein was cannulated with P10 Intramedic polyethylene tubing (BD Biosciences), and 5.8 mg/kg human HRG or an equivalent volume of saline was injected 40 minutes before FeCl3 application. Plasma HRG was quantified by immunoassay. Briefly, plasma was added to wells of a Ni-coated plate (Thermo Scientific) and HRG was detected using an affinity-purified HRG-directed IgG conjugated to horseradish peroxidase.27 In agreement with a previous report,31 the plasma HRG concentration was 1.5 ± 0.5 µM in wild-type mice, and in pilot studies, intravenous administration of 5.8 mg/kg of human HRG to HRG−/− mice resulted in similar plasma HRG concentrations.
RNase (8 mg/kg), DNase (8 mg/kg), or an equivalent volume of saline was injected 15 minutes before FeCl3 application. Conditions in HRG−/− mice (7.5% FeCl3 applied on 1 × 1-mm filter paper for 1 minute) were less stringent than those in wild-type mice (7.5% FeCl3 applied on 1 × 2-mm filter paper for 1.5 minutes)19,33 and were chosen to ensure that thrombosis occurred in a similar time frame in the saline-treated controls. In mice treated with ASOs, thrombosis was induced with 10% FeCl3 applied on 1 × 2-mm filter papers for 1.5 or 1 minute in wild-type and HRG−/− mice, respectively.
Thrombosis was assessed by monitoring blood flow with a T206 ultrasound flow probe (Transonic Systems Inc.) interfaced with a flowmeter and a 0.5-mm flow probe (0.5V series) before FeCl3 application and for 30 minutes thereafter. The time to occlusion (TTO) was calculated as the time required for blood flow to decrease to <20% of the baseline value for at least 3 minutes.34 The area under the plot of blood flow vs time was quantified using SigmaPlot (v.11; SPSS, IBM, Armonk, NY). Thrombi were harvested and subjected to histologic analyses as described in supplemental Methods.
Tail bleeding.
To assess the role of HRG in hemostasis, cumulative blood loss was quantified for 30 minutes after tail-tip amputation as described.35,36 Briefly, after anesthetizing mice with an intraperitoneal injection of 10% ketamine, 5% atropine, and 5% xylazine, tails were transected at a position where the diameter was 1.5 mm, and after immersion in 30 mL of saline prewarmed to 37°C, shed blood was collected for 30 minutes. After subjecting the red blood cells to lysis with ZAP-OGLOBIN II (Beckman Coulter, Mississauga, ON, Canada) and measuring absorbance at 405 nm, hemoglobin was quantified by comparison with a standard curve generated with known amounts of mouse blood.
Surface plasmon resonance (SPR).
See supplemental Methods.
Statistical analysis.
Data are presented as mean ± SD or standard error as indicated. Significance of differences was determined using the Student t test for paired data or analysis of variance (ANOVA; Holm-Sidak method) for group data. For all analyses, P < .05 was considered statistically significant. GraphPad Prism 4 (La Jolla, CA) was used for statistical analyses and for creating scatter diagrams.
Results
Contact activation and thrombin generation are enhanced in plasma from HRG-deficient mice
The dilute PT values in plasma from HRG−/−, HRG+/−, and wild-type mice were similar (supplemental Figure 1A), findings consistent with a previous report of only a 1-second difference between the mean PT in HRG−/− and wild-type mice.31 In contrast, the dilute aPTT in plasma from HRG−/− mice was significantly shorter than that in wild-type mice, and was intermediate in HRG+/− mice (supplemental Figure 1A). To confirm the concept that HRG targets the intrinsic pathway, thrombin generation studies were performed. When initiated with RecombiPlasTin, there were no significant differences in thrombin generation variables in plasma from HRG−/−, HRG+/−, and wild-type mice (supplemental Figure 1B,D). In contrast, when triggered with aPTT reagent, thrombin generation was significantly enhanced in plasma from HRG−/− mice compared with that in wild-type mice, and was intermediate in HRG+/− mice (supplemental Figure 1B-C). Therefore, HRG attenuates clotting initiated via the intrinsic pathway but has no effect on that initiated via the extrinsic pathway, consistent with results obtained in human plasma.27
HRG attenuates RNA- and DNA-induced thrombin generation and clot formation
To determine whether similar abrogation occurs with nucleic acid–induced clotting, we compared RNA- and DNA-induced thrombin generation in plasma from wild-type, HRG+/−, and HRG−/− mice. When initiated with RNA, thrombin generation in plasma from HRG−/− mice was enhanced compared with that in plasma from wild-type mice and intermediate in HRG+/− mice (Figure 1A-D). Thus, the lag time and time to peak thrombin in plasma from HRG−/− mice were 1.5- and twofold shorter, respectively, than those in plasma from wild-type mice (Figure 1A-B), and peak thrombin and endogenous thrombin potential (ETP) were increased by 3.7- and 1.8-fold, respectively (Figure 1C-D). Similar results were observed with DNA as the initiator (Figure 1A-D). The differences in RNA- and DNA-induced clotting in plasma from HRG−/− and wild-type mice were abrogated when HRG-deficient plasma was supplemented with human HRG to 2 µM (Figure 1E-F). Therefore, HRG modulates the capacity of RNA and DNA to activate the intrinsic pathway.
HRG attenuates RNA- and DNA-induced thrombin generation and clotting. (A-D) Thrombin generation in plasma from wild-type, HRG+/−, and HRG−/− mice containing 30 μM PCPS was triggered with RNA (16 μg/mL) or DNA (20 μg/mL) and 15 mM CaCl2. Thrombin generated was quantified by monitoring hydrolysis of Z-Gly-Gly-Arg-AMC. Experiments were performed 3 times in duplicate. *P < .05, **P < .005 for thrombin generation initiated with DNA; ##P < .005 for thrombin initiated with RNA, compared with wild-type plasma. (E-F) Clotting was initiated in plasma from wild-type (black bars) or HRG−/− (gray bars) mice containing human HRG (0-3 μM) by addition of 30 μg/mL of DNA (E) or RNA (F) and 8.3 mM CaCl2. Absorbance was monitored and the clot time was calculated as the time to achieve half maximum absorbance. Bars reflect the mean of 3 experiments, each performed in duplicate, and the lines above the bars reflect the standard deviation (SD). *P < .05 and **P < .005 compared with HRG−/− plasma without added HRG; data were analyzed using Student t tests.
HRG attenuates RNA- and DNA-induced thrombin generation and clotting. (A-D) Thrombin generation in plasma from wild-type, HRG+/−, and HRG−/− mice containing 30 μM PCPS was triggered with RNA (16 μg/mL) or DNA (20 μg/mL) and 15 mM CaCl2. Thrombin generated was quantified by monitoring hydrolysis of Z-Gly-Gly-Arg-AMC. Experiments were performed 3 times in duplicate. *P < .05, **P < .005 for thrombin generation initiated with DNA; ##P < .005 for thrombin initiated with RNA, compared with wild-type plasma. (E-F) Clotting was initiated in plasma from wild-type (black bars) or HRG−/− (gray bars) mice containing human HRG (0-3 μM) by addition of 30 μg/mL of DNA (E) or RNA (F) and 8.3 mM CaCl2. Absorbance was monitored and the clot time was calculated as the time to achieve half maximum absorbance. Bars reflect the mean of 3 experiments, each performed in duplicate, and the lines above the bars reflect the standard deviation (SD). *P < .05 and **P < .005 compared with HRG−/− plasma without added HRG; data were analyzed using Student t tests.
RNA mediates FeCl3 injury-induced arterial thrombosis
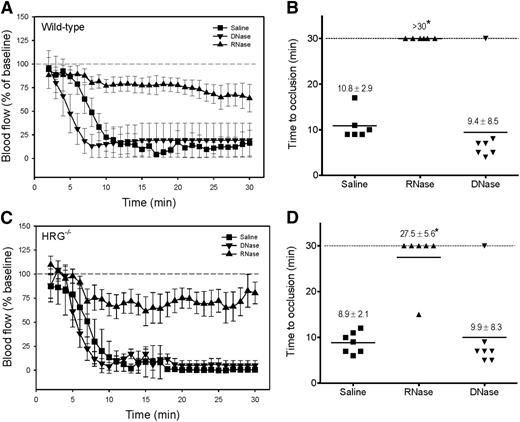
To validate our in vitro observation that HRG modulates activation of the contact system, we set out to examine the role of HRG in a murine model of arterial thrombosis. Although FeCl3 is the most frequently used model, there is conflicting evidence about whether thrombosis in this model is DNA- or RNA-driven.19,37 To address this controversy, we compared the effect of DNase or RNase administration on FeCl3-induced arterial thrombosis in wild-type mice with that of saline. Using conditions that ensured rapid occlusion after FeCl3 application in wild-type mice, the mean TTOs with saline and DNase were similar (10.8 ± 2.9 and 9.4 ± 8.5 min, respectively; P = .7). In contrast, with RNase administration, there was no occlusion after 30 minutes (Figure 2A-B). Similar results were observed in HRG−/− mice (Figure 2C-D). Despite the autofluorescence surrounding the artery, RNA (Figure 3A) was detected in thrombi harvested from FeCl3-treated mice, and RNase administration reduced RNA staining (Figure 3B). In contrast, DNA was not detected (Figure 3C) and DNase administration had no effect (Figure 3D). These results confirm that FeCl3-induced thrombosis is mediated by RNA, and not by DNA.19
RNA initiates thrombosis after FeCl3-induced carotid artery injury. After injection of 8 mg/kg of RNase or DNase, or an equivalent volume of saline, 7.5% FeCl3 was applied to the right carotid arteries of (A) wild-type (1 × 2-mm filter paper for 1.5 minutes) or (C) HRG−/− mice (1 × 1-mm filter paper for 1 minute) to induce vascular injury (n = 6-7/group) and blood flow was continuously monitored and data were plotted vs time. Symbols represent the mean, and the bars reflect the standard error (SE). (B,D) Respective TTO data are presented as a scatter diagram, where symbols represent individual values and horizontal lines represent the mean in each of the 3 groups. Values represent mean ± SD; *P < .001 compared with the saline control group (ANOVA, Holm-Sidak method).
RNA initiates thrombosis after FeCl3-induced carotid artery injury. After injection of 8 mg/kg of RNase or DNase, or an equivalent volume of saline, 7.5% FeCl3 was applied to the right carotid arteries of (A) wild-type (1 × 2-mm filter paper for 1.5 minutes) or (C) HRG−/− mice (1 × 1-mm filter paper for 1 minute) to induce vascular injury (n = 6-7/group) and blood flow was continuously monitored and data were plotted vs time. Symbols represent the mean, and the bars reflect the standard error (SE). (B,D) Respective TTO data are presented as a scatter diagram, where symbols represent individual values and horizontal lines represent the mean in each of the 3 groups. Values represent mean ± SD; *P < .001 compared with the saline control group (ANOVA, Holm-Sidak method).
RNA is detected in thrombi harvested from mice after FeCl3-induced carotid artery injury. Representative thrombi harvested from the carotid artery of wild-type mice pretreated with (A,C) saline, (B) RNase, or (D) DNase. Before injury, mice were infused with an RNA- or DNA-specific fluorescent stain to identify RNA and DNA, respectively, within the thrombus. Thrombi were sectioned and subjected to immunofluorescence analysis using antibodies against HRG, fibrin, or platelets, or were stained with hematoxylin and eosin (H&E). Histologic sections were visualized with a ×100 objective lens fitted to an Olympus BX41 microscope equipped with a DP72 camera. Scale bars represent 100 μm.
RNA is detected in thrombi harvested from mice after FeCl3-induced carotid artery injury. Representative thrombi harvested from the carotid artery of wild-type mice pretreated with (A,C) saline, (B) RNase, or (D) DNase. Before injury, mice were infused with an RNA- or DNA-specific fluorescent stain to identify RNA and DNA, respectively, within the thrombus. Thrombi were sectioned and subjected to immunofluorescence analysis using antibodies against HRG, fibrin, or platelets, or were stained with hematoxylin and eosin (H&E). Histologic sections were visualized with a ×100 objective lens fitted to an Olympus BX41 microscope equipped with a DP72 camera. Scale bars represent 100 μm.
FeCl3-induced arterial thrombosis occurs via the contact system
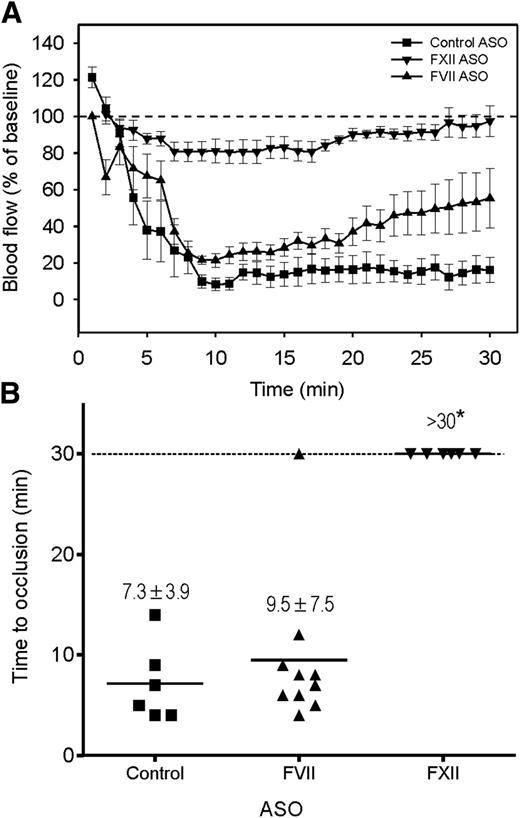
Although others have shown that FeCl3-induced arterial thrombosis is attenuated in FXII-deficient mice, the relative contribution of the intrinsic and extrinsic pathways to thrombosis in this model is uncertain. To investigate this, we used ASOs to lower the levels of FXII or FVII and examined their effect on FeCl3-induced thrombosis in wild-type and HRG−/− mice. Compared with untreated mice and those given control ASO, the FXII and FVII ASOs reduced plasma levels of their cognate proteins by 99% and 90% to 95%, respectively, as determined by immunoblot analysis (supplemental Figure 2A-B). As expected, FXII knockdown significantly (P < .001) prolonged the dilute aPTT two- to threefold but had no effect on the dilute PT (supplemental Figure 2C-D), whereas FVII knockdown significantly (P < .01) prolonged the dilute PT by 1.4- to 1.7-fold but did not influence the dilute aPTT. To ensure rapid occlusion in wild-type mice given the control ASO, FeCl3 was applied with a larger filter paper for a longer time. Whereas the TTO in wild-type mice given the FVII ASO was similar to that in controls (P = .2), the TTO was significantly (P < .001) prolonged to >30 minutes in those given the FXII ASO (Figure 4A-B), findings consistent with the attenuated thrombosis reported in FXII−/− mice,7 but at odds with the reduced thrombosis observed in mice expressing TF on vascular smooth muscle cells.38 Similar results were observed in HRG−/− mice (supplemental Figure 2E-F). Therefore, our results confirm that FXII contributes to arterial thrombosis in the FeCl3 model to a greater extent than FVII. Because the knockdown of FVII was incomplete, we cannot exclude the possibility that the extrinsic pathway contributes to thrombosis in the FeCl3 model.
Thrombosis after FeCl3-induced carotid artery injury occurs mainly via the contact system. Wild-type mice were given FXII, FVII, or control ASO by twice weekly subcutaneous injection for up to 6 weeks. A 1 × 2-mm filter paper presoaked in 10% FeCl3 was applied to the right carotid artery for 1.5 minutes to induce vascular injury (n = 6-10/group). (A) Blood flow was continuously monitored before and for 30 minutes after injury. Symbols represent the mean, and the bars reflect the SE. (B) Blood flow and TTO were determined in wild-type mice pretreated with FXII, FVII, or control ASO. TTO data are presented as a scatter diagram, where symbols represent individual values and horizontal lines represent the mean in each of the 3 groups. Values represent mean ± SD; *P < .001 compared with the control ASO group (ANOVA, Holm-Sidak method).
Thrombosis after FeCl3-induced carotid artery injury occurs mainly via the contact system. Wild-type mice were given FXII, FVII, or control ASO by twice weekly subcutaneous injection for up to 6 weeks. A 1 × 2-mm filter paper presoaked in 10% FeCl3 was applied to the right carotid artery for 1.5 minutes to induce vascular injury (n = 6-10/group). (A) Blood flow was continuously monitored before and for 30 minutes after injury. Symbols represent the mean, and the bars reflect the SE. (B) Blood flow and TTO were determined in wild-type mice pretreated with FXII, FVII, or control ASO. TTO data are presented as a scatter diagram, where symbols represent individual values and horizontal lines represent the mean in each of the 3 groups. Values represent mean ± SD; *P < .001 compared with the control ASO group (ANOVA, Holm-Sidak method).
HRG attenuates FeCl3-induced arterial thrombosis
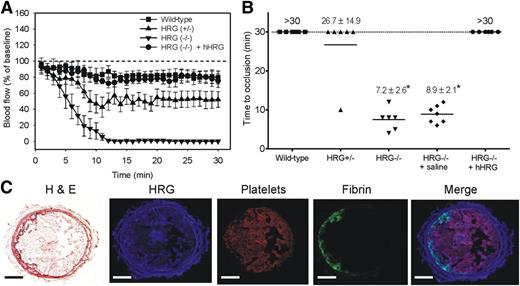
To determine whether HRG deficiency results in a prothrombotic phenotype, we compared FeCl3-induced arterial thrombosis in wild-type mice with that in HRG−/− and HRG+/− mice using milder FeCl3 conditions. Whereas no occlusion was observed for up to 30 minutes in wild-type mice, the mean TTO in HRG−/− mice was 7.2 ± 2.6 minutes treated under the same FeCl3 conditions (Figure 5A-B). Although mean TTO in HRG+/− mice was 26.7 ± 14.9 minutes, blood flow was reduced by 60% within 10 minutes after injury and remained decreased thereafter. Furthermore, the mean area under the blood flow vs time curve was significantly (P < .001) reduced by 1.5- and 7.5-fold in HRG+/− and HRG−/− mice, respectively, compared with that in wild-type mice (1507.4 ± 452.3, 304.4 ± 189.4, and 2252.7 ± 392.9% blood flow ⋅ min, respectively). Therefore, thrombosis is significantly accelerated in HRG−/− mice, an effect also observed in HRG+/− mice. These findings suggest that HRG deficiency results in a prothrombotic phenotype, and even 50% residual HRG is not fully protective.
HRG deficiency is associated with accelerated thrombosis after FeCl3-induced arterial injury. (A) Changes in blood flow after FeCl3-induced carotid artery injury in wild-type, HRG+/−, or HRG−/− mice, and HRG−/− mice given human HRG to 2 μM or saline (n = 6 or 7/group). Thrombosis was initiated by application of a 1 × 1-mm filter paper presoaked in 7.5% FeCl3 for 1 minute to the right carotid artery. Blood flow was continuously measured before and for 30 minutes after injury. Flow measurements at the times indicated were expressed as a percentage of baseline flow. Symbols represent the mean, and the bars reflect the SE. (B) TTO values in wild-type, HRG+/−, or HRG−/− mice, and HRG−/− mice given human HRG or saline. TTO was defined as the time required for blood flow to decrease to <20% of the baseline value for at least 3 consecutive minutes. TTO data are presented as a scatter diagram, where symbols represent individual values, and horizontal lines represent the mean in each of the 5 groups. Values represent mean ± SD; *P < .001 compared with wild-type mice (ANOVA, Holm-Sidak method). (C) Representative thrombus harvested from the carotid artery of a wild-type mouse 30 minutes after FeCl3-induced injury and subjected to immunofluorescence analysis using antibodies against HRG, platelets, or fibrin, or stained with hematoxylin and eosin (H&E). Histologic sections were visualized using a ×100 objective lens fitted to an Olympus BX41 microscope equipped with a DP72 camera. Scale bars represent 100 μm.
HRG deficiency is associated with accelerated thrombosis after FeCl3-induced arterial injury. (A) Changes in blood flow after FeCl3-induced carotid artery injury in wild-type, HRG+/−, or HRG−/− mice, and HRG−/− mice given human HRG to 2 μM or saline (n = 6 or 7/group). Thrombosis was initiated by application of a 1 × 1-mm filter paper presoaked in 7.5% FeCl3 for 1 minute to the right carotid artery. Blood flow was continuously measured before and for 30 minutes after injury. Flow measurements at the times indicated were expressed as a percentage of baseline flow. Symbols represent the mean, and the bars reflect the SE. (B) TTO values in wild-type, HRG+/−, or HRG−/− mice, and HRG−/− mice given human HRG or saline. TTO was defined as the time required for blood flow to decrease to <20% of the baseline value for at least 3 consecutive minutes. TTO data are presented as a scatter diagram, where symbols represent individual values, and horizontal lines represent the mean in each of the 5 groups. Values represent mean ± SD; *P < .001 compared with wild-type mice (ANOVA, Holm-Sidak method). (C) Representative thrombus harvested from the carotid artery of a wild-type mouse 30 minutes after FeCl3-induced injury and subjected to immunofluorescence analysis using antibodies against HRG, platelets, or fibrin, or stained with hematoxylin and eosin (H&E). Histologic sections were visualized using a ×100 objective lens fitted to an Olympus BX41 microscope equipped with a DP72 camera. Scale bars represent 100 μm.
HRG supplementation abrogates the prothrombotic phenotype in HRG−/− mice
To further validate the role of HRG in this model, HRG−/− mice were given HRG or saline before FeCl3-induced injury. Whereas the mean TTO with saline was 8.9 ± 2.1 minutes, the mean TTO in mice supplemented with HRG was >30 minutes (Figure 5A-B). Therefore, restoration of HRG to levels similar to those in wild-type mice abrogates the prothrombotic phenotype in HRG−/− mice.
Immunostaining localizes HRG with platelets and fibrin in thrombi formed after FeCl3-induced injury
To localize HRG, thrombi from FeCl3-treated wild-type mice were harvested and subjected to immunohistochemical analysis. Whereas platelets were dispersed throughout the thrombi, fibrin deposition was localized to the vessel wall, likely at the site of FeCl3 application (Figure 5C). HRG is reported to be stored in platelet α-granules and released upon platelet activation. Because HRG binds to fibrin,28,39 we hypothesized that HRG would localize with platelets and fibrin. Consistent with this, HRG in thrombi localized with platelets and, to a lesser extent, with fibrin (Figure 5C). These data confirm that HRG is found in thrombi formed after FeCl3-induced arterial injury in mice.
Hemostasis is normal in HRG−/− or HRG+/− mice
To examine the role of HRG in hemostasis, tail bleeding in HRG−/−, HRG+/−, and wild-type mice was compared. Cumulative blood loss after tail transection (Figure 6) was not significantly different in HRG−/− and HRG+/− mice from that in the wild-type controls (24.9 ± 3.9, 19.1 ± 3.1, and 26.5 ± 4.9 μL, respectively). These findings are at odds with previous work, which reported a modestly shorter bleeding time in HRG−/− mice than in wild-type mice.31 The discrepancy may reflect differences in bleeding models; we standardized the site of tail transection at 1.5 mm and used the more sensitive measure of cumulative blood loss instead of bleeding time. Overall, our results suggest that HRG modulates thrombosis with minimal effects on hemostasis.
HRG deficiency has no impact on hemostasis. Cumulative blood loss was quantified for 30 minutes after tail-tip amputation in wild-type, HRG+/−, and HRG−/− mice (n = 9-11/group). Symbols represent individual values, whereas horizontal lines represent the mean in each of the 3 groups. Values represent mean ± SD.
HRG deficiency has no impact on hemostasis. Cumulative blood loss was quantified for 30 minutes after tail-tip amputation in wild-type, HRG+/−, and HRG−/− mice (n = 9-11/group). Symbols represent individual values, whereas horizontal lines represent the mean in each of the 3 groups. Values represent mean ± SD.
HRG binds to DNA and RNA
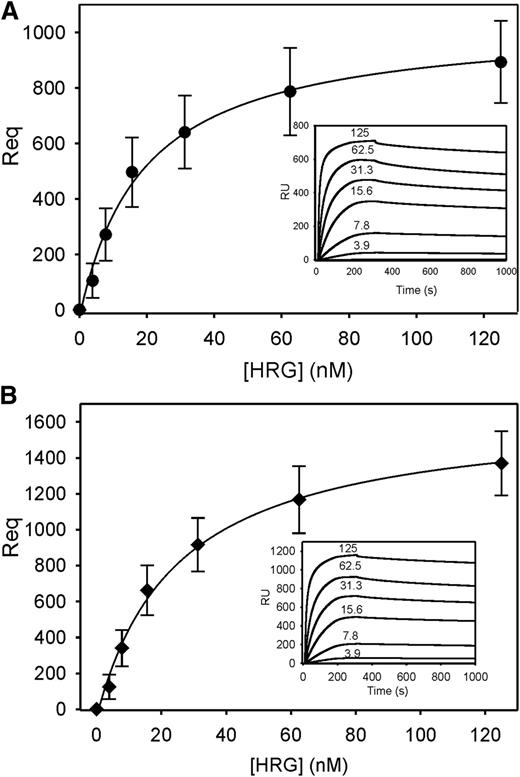
To determine whether HRG binds to nucleic acids, SPR was used to quantify the binding of HRG to immobilized biotinylated DNA or RNA. The sensorgrams revealed rapid association and slow dissociation of HRG from biotin DNA and biotin RNA (Figure 7, insets). Plots of response unit (RU) values at equilibrium (Req) vs input HRG concentrations showed that HRG binds to DNA and RNA in a concentration-dependent and saturable fashion (Figure 7). Kinetic analysis of the on- and off-rates of the sensorgrams demonstrated that HRG binds to both nucleic acids with identical Kd values of 1.3 ± 0.8 nM. The high affinity of HRG for DNA supports previous work40 and, collectively, these observations provide a potential mechanism by which nucleic acids induce contact activation of coagulation, and HRG attenuates this process.
Binding of HRG to DNA or RNA as determined using SPR. (A) Biotin DNA or (B) biotin RNA was adsorbed to 200 to 300 RU on separate flow cells containing immobilized streptavidin. An unmodified streptavidin-containing cell served as the reference control. After injecting 0 to 0.125 nM HRG into flow cells for 300 seconds to assess binding, buffer was injected for 800 seconds to assess dissociation. The amount of HRG bound at equilibrium (Req) was corrected for background and plotted vs input HRG concentrations. Data represent the mean ± SD of 3 determinations and were fit by nonlinear regression analysis using a rectangular hyperbola (line). Insets, representative binding sensorgrams for DNA and RNA, where input HRG concentrations are indicated adjacent to each tracing.
Binding of HRG to DNA or RNA as determined using SPR. (A) Biotin DNA or (B) biotin RNA was adsorbed to 200 to 300 RU on separate flow cells containing immobilized streptavidin. An unmodified streptavidin-containing cell served as the reference control. After injecting 0 to 0.125 nM HRG into flow cells for 300 seconds to assess binding, buffer was injected for 800 seconds to assess dissociation. The amount of HRG bound at equilibrium (Req) was corrected for background and plotted vs input HRG concentrations. Data represent the mean ± SD of 3 determinations and were fit by nonlinear regression analysis using a rectangular hyperbola (line). Insets, representative binding sensorgrams for DNA and RNA, where input HRG concentrations are indicated adjacent to each tracing.
Discussion
Emerging data highlight the importance of the contact system in the pathogenesis of thrombosis and identify naturally-occurring polyphosphates, such as nucleic acids, as potential initiators of this pathway. However, it is unclear how the contact system is regulated to prevent uncontrolled clotting. Previously, we demonstrated that HRG binds FXIIa with high affinity and attenuates contact activation of coagulation in plasma.27 We now show that: (1) HRG-deficient mice have similar PT but shorter aPTT values than their wild-type counterparts, thereby localizing the in vivo HRG effect to the intrinsic pathway; (2) DNA- and RNA-induced thrombin generation is enhanced in plasma from HRG-deficient mice; (3) HRG binds DNA and RNA with high affinity, a finding consistent with the concept that HRG modulates nucleic acid–induced coagulation; and (4) although tail bleeding in HRG−/− and wild-type mice is similar, thrombosis is accelerated in HRG−/− mice, a difference abrogated with HRG administration. Therefore, these findings suggest that HRG deficiency induces a prothrombotic phenotype without influencing hemostasis.
Several lines of evidence suggest that the modulating effect of HRG on coagulation occurs via the intrinsic pathway. First, HRG binds DNA and RNA and attenuates DNA- and RNA-induced thrombin generation, which is triggered by FXII activation. Second, FeCl3-induced thrombosis is accelerated in HRG-deficient mice, a model that appears to be driven, at least in part, by RNA and is dependent on FXII. Although some reports suggest that DNA contributes to FeCl3-induced thrombosis,37 HRG is a universal modulator because it attenuates coagulation regardless of whether it is initiated by DNA or RNA. Third, HRG has little or no effect on hemostasis, which is mediated primarily by the extrinsic pathway.
The origin of RNA in the FeCl3 model is currently unknown. There are conflicting data about whether FeCl3 induces endothelial denudation or triggers thrombosis through other mechanisms.41,42 A recent report implicates red blood cells in the pathogenesis of FeCl3-induced thrombosis,42 and in vitro studies suggest that when activated, red blood cells promote thrombin generation.43
DNA in neutrophil extracellular traps and histones promote clotting in vitro and may contribute to thrombosis in murine models of deep-vein thrombosis and stroke.21,22,44,45 The role of DNA under the conditions of our FeCl3 model is uncertain because DNase administration did not influence arterial occlusion, and we were unable to detect DNA in thrombi. Taken together, the evidence thus far suggests that the type of nucleic acid released into the blood may vary depending on the method used to induce vascular injury.
Once HRG binds to FXIIa, the capacity of FXIIa to propagate coagulation through autoactivation of FXII and activation of FXI is attenuated.27 Platelets are reported to store zinc and HRG in their α-granules, and both components are released upon platelet activation.39,46 In the presence of zinc, the affinity of FXIIa for HRG is heightened by 1000-fold to a Kd value of 7.5 pM,27 which is higher than the affinity of HRG for other ligands.26 This creates a potential molecular switch that directs HRG to FXIIa. Given the abundance of HRG and zinc in platelets, HRG is more likely to bind FXIIa in the vicinity of platelet-rich thrombi than is C1-inhibitor, the putative physiological regulator of FXIIa.27 Furthermore, we have shown that HRG binds DNA and RNA with Kd values of ∼1 nM, which localizes HRG to the site of contact activation, where it is poised to modulate FXIIa activity. Although FXIIa is protected from inhibition by C1-inhibitor when it associates with polyanionic surfaces,47 HRG retains its capacity to modulate FXIIa activity, even when the protease is associated with nucleic acids.48 Consequently, HRG is likely to be a more important modulator of FXIIa activity than C1-inhibitor, a concept supported by the enhanced DNA- and RNA-induced thrombin generation observed in plasma from HRG-deficient mice, which is attenuated with HRG supplementation. Therefore, by acting as a molecular brake to limit the procoagulant response, HRG is poised to serve as a dynamic regulator of the contact system, regardless of whether it is initiated by DNA or RNA. This is an important concept because the identification of several novel physiologic activators of the contact system demands a compensatory regulatory mechanism.
Authorship
Contribution: T.T.V. designed and performed experiments, analyzed data, and wrote the paper; J.Z., B.A.L., and A.R.S. designed and performed experiments and analyzed data; R.N., S.Q., and N.V. performed experiments; W.J.-D. and B.P.M., provided vital reagents; P.L.G. designed experiments and analyzed data; and J.C.F. and J.I.W. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: B.P.M. is an employee of Isis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey I. Weitz, Thrombosis and Atherosclerosis Research Institute, 237 Barton St E, Hamilton, ON L8L 2X2, Canada; e-mail:weitzj@taari.ca.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Zakhar Lysov for assistance with cell culture.
J.I.W. holds the Heart and Stroke Foundation/J. Fraser Mustard Endowed Chair in Cardiovascular Research and the Canada Research Chair (Tier 1) in Thrombosis.
This work was supported in part by the Canadian Institutes of Health Research (FRN-392, MOP-136820, CTP 79846), the Heart and Stroke Foundation (T6357), and the Ontario Research and Development Challenge Fund; a Natural Sciences and Engineering Research Council of Canada, Canada Graduate Scholarships-Doctoral Award (T.T.V.); and Ontario Graduate Scholarships (R.N., N.V.).
References
Author notes
T.T.V. and J.Z. contributed equally to this study and are considered cofirst authors.