Key Points
Ibrutinib was well tolerated when administered with BR CIT in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma.
Ibrutinib added to CIT was associated with a high degree of clinical activity that compares favorably to historical reports of CIT alone.
Abstract
The safety and efficacy of ibrutinib, an oral inhibitor of Bruton tyrosine kinase, were evaluated with chemoimmunotherapy (CIT) in a multicenter phase 1b study. Patients with relapsed/refractory chronic lymphocytic leukemia received bendamustine and rituximab (BR) or fludarabine, cyclophosphamide, and rituximab (FCR) for up to 6 cycles with daily ibrutinib (420 mg) until progressive disease or unacceptable toxicity. Enrollment to FCR-ibrutinib closed early due to a lack of fludarabine-naïve previously treated patients. No patients treated with BR-ibrutinib (n = 30) or FCR-ibrutinib (n = 3) experienced prolonged hematologic toxicity in cycle 1 (primary end point). Tolerability was as expected with either CIT or single-agent ibrutinib. The overall response rate (ORR) with BR-ibrutinib was 93.3%, including 16.7% complete responses (CRs) initially, which increased to 40% with the extension period. Including 1 patient with partial response with lymphocytosis, the best ORR was 96.7%. Sixteen of 21 patients with baseline cytopenias had sustained hematologic improvement. At 12 and 36 months, 86.3% and 70.3% remained progression-free, respectively. All 3 patients treated with ibrutinib-FCR achieved CR. Ibrutinib may enhance CIT efficacy without additive toxicities, providing the rationale for studying this combination in an ongoing phase 3 trial. The study is registered to www.clinicaltrials.gov as #NCT01292135.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in Western countries and typically occurs in older people; median age at diagnosis is 72 years.1 CLL has a widely variable disease course: some patients have indolent disease not requiring treatment for decades, whereas others have aggressive disease requiring immediate treatment.1,2 Although oral alkylating agents like chlorambucil were historically the initial standard therapy for patients with CLL/small lymphocytic lymphoma (SLL),3 newer agents such as fludarabine, with or without cyclophosphamide,4-6 and bendamustine7 have improved response rates and remission durations. Furthermore, as demonstrated by the German CLL Study Group CLL8 trial, the addition of rituximab to fludarabine and cyclophosphamide (FCR) improved not just progression-free survival (PFS) but also overall survival, establishing chemoimmunotherapy (CIT) as the standard of care for first-line treatment of young and/or fit patients.8-12 Similarly, the REACH trial demonstrated longer PFS with FCR vs fludarabine plus cyclophosphamide in the relapsed setting.11 More recently, phase 2 trials of bendamustine in combination with rituximab (BR) have demonstrated good tolerability and efficacy, with a reported overall response rate (ORR) of 59% and complete remissions achieved in 9%, in the relapsed setting.13 Despite these improvements in ORR, PFS, and even overall survival in treatment-naïve patients, patients continue to relapse and require subsequent therapy. Because subsequent remission duration generally shortens after each CIT, there is a need for new therapies that act via novel targets and can be administered to patients with comorbidities to achieve longer remissions.
B-cell receptor (BCR) signaling and the tissue microenvironment play important roles in the pathogenesis of CLL/SLL.14-21 Downstream of the BCR is Bruton tyrosine kinase (BTK),15 a signal-transduction kinase that plays a critical role in BCR signaling and B-cell development and function.22,23 Loss of BTK in the human disease Bruton X-linked agammaglobulinemia results in the absence of B cells and profound hypogammaglobulinemia. The survival of many B-cell malignancies is dependent on BTK-mediated signals from the BCR.15,22-27 As such, selective BTK inhibition is an attractive approach for diseases driven by BCR activation, including malignancies such as CLL/SLL.28,29
Ibrutinib is a first-in-class once-daily orally administered potent inhibitor of BTK.30 Ibrutinib binds covalently to a cysteine residue (Cys-481) in the BTK active site, resulting in sustained inhibition of the enzyme.31 Previous studies have demonstrated that single-agent ibrutinib is well tolerated and induces high levels of objective responses in patients with B-cell malignancies, including CLL/SLL.32-34 Specifically, a phase 1b/2 study of 85 patients with relapsed/refractory CLL/SLL showed a high investigator-assessed ORR (71%), with 75% of patients progression-free at 26 months.33 Furthermore, this study showed that long-term therapy with ibrutinib was associated with only modest toxicity, with predominantly grade 1 or 2 adverse events (AEs).33 Ibrutinib was approved in patients who received at least 1 prior therapy, initially for the treatment of mantle cell lymphoma and more recently for CLL.35 Because single-agent ibrutinib has shown good tolerability, we sought to combine it with the 2 most standard CIT regimens in CLL, with the goal of achieving more prolonged disease control. To this end, the current study evaluated the safety and efficacy of ibrutinib in combination with standard BR or FCR in patients with relapsed/refractory CLL/SLL.
Patients and methods
Patient eligibility
This study (#NCT01292135; www.clinicaltrials.gov) was performed in accordance with the ethical principles that originate in the Declaration of Helsinki and that are consistent with the International Conference on Harmonization/Good Clinical Practice and applicable regulatory requirements. The protocol was institutional review board approved at all sites, and all patients provided written informed consent. The data were analyzed by F.C., D.F.J., and T.G., and all authors had access to the primary data.
This open-label PCYC-1108 study enrolled patients into 2 parallel cohorts, BR-ibrutinib and FCR-ibrutinib. Eligible patients had a confirmed diagnosis of either CLL or SLL; had received between 1 and 3 prior treatment regimens; met International Workshop on Chronic Lymphocytic Leukemia 2008 criteria for needing therapy36 ; were >18 years old; had Eastern Cooperative Oncology Group performance status ≤1; and were purine analog–naïve (for FCR cohort only). Exclusion criteria included Richter transformation; any life-threatening illness or medical condition that could compromise safety or interfere with the absorption or metabolism of ibrutinib; and laboratory abnormalities including absolute neutrophil count (ANC) <1000 cells per mm3 (1.0 × 109/L), platelet count <50 000 per mm3 (50 × 109/L), serum aspartate transaminase or alanine transaminase ≥3.0 × upper limit of normal, creatinine >2.0 × upper limit of normal, or creatinine clearance <40 mL/min.
Treatment plan and clinical assessment
All patients received ibrutinib (420 mg) once daily starting on the first day of the cycle. One cycle of therapy consisted of 28 days. Ibrutinib was administered orally after CIT infusions. Treatment with ibrutinib continued daily, in 28-day cycles until disease progression or unacceptable toxicity. Prophylactic antibiotics and/or growth factors were not required per protocol but were allowed at the investigator’s discretion. After at least 12 cycles, patients could enroll in the ongoing long-term extension study (PCYC-1103) to continue ibrutinib therapy and follow-up. Patients in each treatment group were enrolled in parallel cohorts and analyzed independently. Enrollment to the FCR-ibrutinib group was suspended due to slow accrual of relapsed patients who were purine analog–naïve and who were candidates for FCR. The 3 patients enrolled in the FCR combination group continued to receive treatment and assessments per protocol.
BR or FCR was administered intravenously for a maximum of 6 cycles. BR comprised bendamustine 70 mg/m2 on days 1 and 2 of each cycle. FCR comprised fludarabine 25 mg/m2 per day and cyclophosphamide 250 mg/m2 per day for 3 days (days 1-3) of each cycle, repeated every 28 days. Rituximab was administered similarly in both cohorts. For cycle 1 only, rituximab was given at 375 mg/m2 on day 1; alternatively, the rituximab dose could be divided between day 1 and day 2 per institutional standards for patients considered at risk for infusion reactions. In subsequent cycles of therapy, rituximab was given at a dose of 500 mg/m2 on day 1. FCR or BR treatment could be stopped sooner than 6 cycles in patients with unacceptable toxicity or disease progression. Ibrutinib monotherapy could continue indefinitely until progression or unacceptable toxicity. Response assessment included a computed tomography (CT) radiologic examination, and complete response (CR) was confirmed by a bone marrow biopsy and CT.36 Tumor assessments (based on serially collected CT scans) and bone marrow assessments were source verified by the clinical monitors.
Study end points
The primary end point of the study was the incidence of prolonged hematologic toxicity starting in cycle 1, which was defined as either grade ≥3 neutropenia lasting for ≥6 weeks (ie, not improving to grade ≤2) after the start of any study medication (ibrutinib, BR, or FCR) or grade ≥3 thrombocytopenia lasting for ≥8 weeks (ie, not improving to grade ≤2) after the start of any study medication. In PCYC-1108, all grade AEs were collected, whereas in PCYC-1103, only AEs grade ≥3, serious AEs, and those leading to dose modification or discontinuation were captured. Secondary safety end points included the incidence of AEs requiring dose delay or discontinuation of ibrutinib, overall incidence of grade ≥3 AEs, and overall incidence of serious AEs. Secondary clinical end points for this study were ORR (CR, CR with incomplete marrow recovery [CRi], nodular partial response [nPR], and partial response [PR]); hematologic improvement in patients with neutropenia, anemia, or thrombocytopenia at baseline; and PFS.
Statistical considerations
A total of 30 patients were enrolled in the study to receive BR-ibrutinib and contributed to the overall safety and efficacy analyses. Due to lack of enrollment, the FCR-ibrutinib group was limited to 3 patients and their results are summarized separately. Patient demographics and other baseline characteristics have been summarized, with summary statistics including means, standard deviations, and medians for continuous variables, as well as proportions for categorical variables. To assess the primary end point of prolonged hematologic toxicity in cycle 1, the proportion of patients with prolonged hematologic toxicity with a corresponding 2-sided exact binomial 95% confidence interval was calculated.
The number and proportion of patients achieving a CR, CRi, nPR, or PR, along with a 95% exact binomial confidence interval were determined, and the proportion of patients who achieved a best overall response of CR, CRi, nPR, PR, or PR with lymphocytosis (PR-L) is summarized. For patients who began the study with neutropenia (baseline ANC ≤1500/μL), anemia (baseline hemoglobin ≤11 g/dL), or thrombocytopenia (baseline platelet count ≤100 000/μL), hematologic improvement was defined as an improvement ≥50% above baseline or an increase to >1500/μL, >11 g/dL, or >100 000/μL for ANC, hemoglobin, or platelets, respectively, maintained for ≥8 weeks for the baseline cytopenic parameter. Kaplan-Meier methodology was used to estimate the durability of response measured by landmark rates and the PFS curves with corresponding quartiles (including the median). No imputation of missing values for tumor assessment data, laboratory data, vital signs, physical examination, or electrocardiographic data was performed.
Results
Patient characteristics: BR cohort
Thirty patients were enrolled to receive BR-ibrutinib. The median age was 62 years (range: 41-82 years) and 83.3% were men (Table 1). The median number of prior regimens was 2 (range: 1-3). Median time from the start of most recent prior therapy to first dose was 15.8 months. Of note, all patients had received prior rituximab treatment and prior systemic chemotherapy; purine analogs (90%) and alkylating agents (86.7%) were the most common types of prior chemotherapy. Eleven patients (36.7%) were purine analog–refractory (defined as treatment-free interval <12 months following a purine analog regimen), with 6 of these patients (20%) having treatment-free intervals <6 months. In addition, 4 patients (13.3%) were bendamustine–refractory (defined as treatment-free interval <12 months following a bendamustine-containing regimen), with all 4 patients having treatment-free intervals <6 months. At the time of study enrollment, 16 patients (53.3%) had advanced Rai stage III/IV disease, and 16 (53.3%) had bulky disease (lymph nodes ≥5 cm). High-risk cytogenetics were common, with 7 patients (23.3%) and 10 patients (33.3%) carrying del17p and del11q, respectively (Table 1).
Baseline patient characteristics, BR-ibrutinib cohort (N = 30)
| Male, n (%) | 25 (83.3) |
| Median age, y (range) | 62 (41-82) |
| Median time from initial diagnosis to first dose, mo (range) | 78.6 (7.6-159.6) |
| ECOG performance status, n (%) | |
| 0 | 16 (53.3) |
| 1 | 14 (46.7) |
| Median number of prior treatment regimens for CLL/SLL | 2 |
| Number of prior regimens, n (%) | |
| 1 | 8 (26.7) |
| 2 | 9 (30.0) |
| 3 | 13 (43.3) |
| Prior therapies for CLL/SLL, n (%) | |
| Rituximab | 30 (100.0) |
| Purine analogs | 27 (90.0) |
| Cyclophosphamide | 24 (80.0) |
| Ofatumumab | 5 (16.7) |
| Bendamustine | 4 (13.3) |
| Alemtuzumab | 4 (13.3) |
| Anthracyclines | 2 (6.7) |
| Lenalidomide | 2 (6.7) |
| High-risk Rai stage (III-IV), n (%) | 16 (53.3) |
| Bulky disease (lymph nodes at least 5 cm), n (%) | 16 (53.3) |
| Presence of cytopenias at enrollment*, n (%) | |
| Neutropenia | 8 (26.7) |
| Anemia | 7 (23.3) |
| Thrombocytopenia | 14 (46.7) |
| FISH/cytogenetics,† n (%) | |
| Del 17p | 7 (23.3) |
| Del 11q | 10 (33.3) |
| Trisomy12 | 3 (10.0) |
| Del 13q (0-1 copy) | 7 (23.3) |
| Del 6q | 2 (6.7) |
| Median serum β2–microglobulin, mg/L (range) | 4.4 (1.7-15.3) |
| Median creatinine clearance, mL/min (range) | 89.0 (46.1-205.8) |
| Creatinine clearance <60 mL/min, n (%) | 4 (13.3) |
| Male, n (%) | 25 (83.3) |
| Median age, y (range) | 62 (41-82) |
| Median time from initial diagnosis to first dose, mo (range) | 78.6 (7.6-159.6) |
| ECOG performance status, n (%) | |
| 0 | 16 (53.3) |
| 1 | 14 (46.7) |
| Median number of prior treatment regimens for CLL/SLL | 2 |
| Number of prior regimens, n (%) | |
| 1 | 8 (26.7) |
| 2 | 9 (30.0) |
| 3 | 13 (43.3) |
| Prior therapies for CLL/SLL, n (%) | |
| Rituximab | 30 (100.0) |
| Purine analogs | 27 (90.0) |
| Cyclophosphamide | 24 (80.0) |
| Ofatumumab | 5 (16.7) |
| Bendamustine | 4 (13.3) |
| Alemtuzumab | 4 (13.3) |
| Anthracyclines | 2 (6.7) |
| Lenalidomide | 2 (6.7) |
| High-risk Rai stage (III-IV), n (%) | 16 (53.3) |
| Bulky disease (lymph nodes at least 5 cm), n (%) | 16 (53.3) |
| Presence of cytopenias at enrollment*, n (%) | |
| Neutropenia | 8 (26.7) |
| Anemia | 7 (23.3) |
| Thrombocytopenia | 14 (46.7) |
| FISH/cytogenetics,† n (%) | |
| Del 17p | 7 (23.3) |
| Del 11q | 10 (33.3) |
| Trisomy12 | 3 (10.0) |
| Del 13q (0-1 copy) | 7 (23.3) |
| Del 6q | 2 (6.7) |
| Median serum β2–microglobulin, mg/L (range) | 4.4 (1.7-15.3) |
| Median creatinine clearance, mL/min (range) | 89.0 (46.1-205.8) |
| Creatinine clearance <60 mL/min, n (%) | 4 (13.3) |
ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization.
Neutropenia was defined as baseline ANC ≤1500/μL, anemia was defined as baseline hemoglobin ≤11.0 g/dL, and thrombocytopenia was defined as baseline platelet count ≤100 000/μL.
Based on Dohner hierarchy for FISH cytogenetics.
Patient disposition: BR cohort
The median number of cycles of BR administered was 6 (range: 2-6). Median treatment duration for ibrutinib was 15.7 months and median study follow-up was 15.8 months on the primary study, increasing to 35.4 and 37.3 months, respectively, with follow-up on the extension study. The primary study closed on July 3, 2013. At that time, 21 patients (70%) who were still receiving ibrutinib monotherapy after completion of BR rolled over to a long-term extension study to continue treatment with ibrutinib. Nine patients (30%) discontinued study treatment during the primary study, of whom 5 (16.6%) planned to proceed to stem cell transplant, and 4 (13.3%) discontinued due to progressive disease. At the time of primary study closure, 2 patients had died within the study observation period: 1 discontinued study treatment for a stem cell transplant and later died of sepsis, and the other progressed on treatment and died after initiation of subsequent anticancer therapy. To date, at a median follow-up of 37.3 months, 16 patients (53.3%) continue ibrutinib treatment within the extension study. Eighteen patients (60%) received ibrutinib treatment for ≥2 years. The reasons for treatment discontinuation for the primary and extension study periods are shown in supplemental Table 1, available on the Blood Web site.
Safety: BR cohort
The planned phase 1b safety pause after 6 patients were enrolled revealed no significant safety concerns. The incidence of prolonged cytopenia in cycle 1, including both neutropenia and thrombocytopenia, was 0. Over time, 2 patients (6.7%) experienced grade 3 prolonged hematologic toxicity (1 neutropenia and 1 thrombocytopenia on days 147 and 86, respectively) and 2 additional patients (6.7%) experienced grade 4 prolonged hematologic toxicity (neutropenia on days 143 and 195, respectively); none of these patients discontinued treatment as a result of these toxicities. Fifteen patients (50%) received G-CSF (pegfilgrastim or filgrastim) at some point during the primary study. The median number of BR cycles delivered was 6. No ibrutinib dose reductions were performed (defined as taking less than 420 mg for more than 2 consecutive days). A total of 6 patients did not receive the prespecified 6 cycles of BR (2 patients discontinued bendamustine only) due to toxicities, including neutropenia (in 3 patients after 3-4 cycles); fatigue (in 1 patient after cycle 2); thrombocytopenia (in 1 patient after cycle 5); and fatigue, hot flashes, and myalgia (in 1 patient after 3 cycles). All patients continued to receive ibrutinib.
Over the course of the primary study, the most frequently reported treatment-emergent AEs were diarrhea, nausea, fatigue, neutropenia, and upper respiratory tract infection (Table 2). Overall, the AEs are similar to what is observed with ibrutinib (eg, diarrhea) or BR (eg, neutropenia) administered individually. The majority of treatment-emergent AEs were grade 1 or 2. The most frequently reported treatment-emergent AEs grade ≥3 were neutropenia (40%), maculopapular rash and fatigue (10% each), and thrombocytopenia, febrile neutropenia, and cellulitis (6.7% each). All AEs grade ≥3 occurring over the primary and extension study periods are shown in supplemental Table 2; 1 additional patient experienced an event of grade ≥3 neutropenia, and 1 patient experienced a grade 3 gastrointestinal hemorrhage (occurring 821 days from initiation of study treatment and which resolved in 5 days.) During the primary study, 5 patients (16.7%) experienced grade 3 infections, which included cellulitis (2 patients), herpes zoster (1 patient), sinusitis (1 patient), upper respiratory tract infection (1 patient), and urinary tract infection (1 patient). No additional patients experienced grade ≥3 infections during the extension study period (supplemental Table 2). No events of atrial fibrillation were reported over the course of the primary or long-term extension study periods.
Incidence (≥15%) of treatment-emergent AEs in the primary study (PCYC-1108), BR-ibrutinib cohort (N = 30)
| AE . | Any grade event, n (%) . | Grade ≥3, n (%) . |
|---|---|---|
| Hematologic AEs | ||
| Neutropenia | 12 (40.0) | 12 (40.0) |
| Febrile neutropenia | 2 (6.7) | 2 (6.7) |
| Thrombocytopenia | 5 (16.7) | 2 (6.7) |
| Nonhematologic AEs | ||
| Diarrhea | 21 (70.0) | 1 (3.3) |
| Nausea | 20 (66.7) | — |
| Fatigue | 14 (46.7) | 3 (10.0) |
| Upper respiratory tract infection | 11 (36.7) | 1 (3.3) |
| Edema, peripheral | 10 (33.3) | — |
| Eye disorders | 9 (30.0) | — |
| Constipation | 9 (30.0) | — |
| Vomiting | 9 (30.0) | 1 (3.3) |
| Headache | 9 (30.0) | — |
| Sinusitis | 8 (26.7) | 1 (3.3) |
| Arthralgia | 8 (26.7) | — |
| Pyrexia | 7 (23.3) | — |
| Muscle spasms | 7 (23.3) | — |
| Dizziness | 7 (23.3) | — |
| Insomnia | 7 (23.3) | — |
| Gastroesophageal reflux disease | 6 (20.0) | — |
| Contusion | 6 (20.0) | — |
| Squamous cell carcinoma | 6 (20.0) | — |
| Maculopapular rash | 5 (16.7) | 3 (10.0) |
| Chills | 5 (16.7) | — |
| Decreased appetite | 5 (16.7) | — |
| Back pain | 5 (16.7) | — |
| Myalgia | 5 (16.7) | — |
| AE . | Any grade event, n (%) . | Grade ≥3, n (%) . |
|---|---|---|
| Hematologic AEs | ||
| Neutropenia | 12 (40.0) | 12 (40.0) |
| Febrile neutropenia | 2 (6.7) | 2 (6.7) |
| Thrombocytopenia | 5 (16.7) | 2 (6.7) |
| Nonhematologic AEs | ||
| Diarrhea | 21 (70.0) | 1 (3.3) |
| Nausea | 20 (66.7) | — |
| Fatigue | 14 (46.7) | 3 (10.0) |
| Upper respiratory tract infection | 11 (36.7) | 1 (3.3) |
| Edema, peripheral | 10 (33.3) | — |
| Eye disorders | 9 (30.0) | — |
| Constipation | 9 (30.0) | — |
| Vomiting | 9 (30.0) | 1 (3.3) |
| Headache | 9 (30.0) | — |
| Sinusitis | 8 (26.7) | 1 (3.3) |
| Arthralgia | 8 (26.7) | — |
| Pyrexia | 7 (23.3) | — |
| Muscle spasms | 7 (23.3) | — |
| Dizziness | 7 (23.3) | — |
| Insomnia | 7 (23.3) | — |
| Gastroesophageal reflux disease | 6 (20.0) | — |
| Contusion | 6 (20.0) | — |
| Squamous cell carcinoma | 6 (20.0) | — |
| Maculopapular rash | 5 (16.7) | 3 (10.0) |
| Chills | 5 (16.7) | — |
| Decreased appetite | 5 (16.7) | — |
| Back pain | 5 (16.7) | — |
| Myalgia | 5 (16.7) | — |
Dashes represent the absence of grade 3 or higher AEs.
Other grade 4 events included neutropenia in 9 patients (30%) and hyperuricemia in 1 (3.3%). Six serious AEs (20% of patients) were reported and included 1 event of tumor lysis (3.3%), which required hospital admission; 2 events of cellulitis (6.7%); 1 event of dehydration (3.3%); and 2 events of febrile neutropenia (6.7%). No grade 5 AEs were observed and no patient discontinued ibrutinib therapy due to an AE during the primary study. During the long-term extension period, only 2 patients discontinued treatment due to AEs, both resulting from ovarian cancer (1 fatal event). Over the 3-year follow-up including the extension period, 11 patients experienced other malignancies of any grade, including skin cancer (8 patients), ovarian cancer (2 patients), lung cancer (1 patient), grade 1 neoplasm (not specified, 1 patient), and thyroid neoplasm (1 patient).
Efficacy: BR cohort
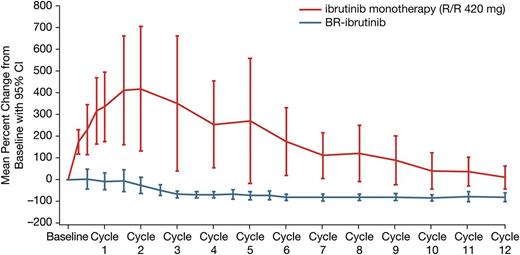
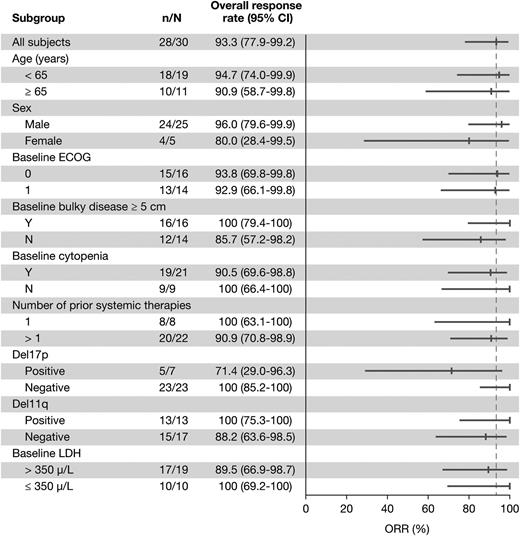
With a median treatment duration of 15.7 months, the ORR was 93.3% (28/30 patients), including 5 (16.7%) CRs, 3 (10%) nPRs, and 20 (66.7%) PRs. One additional patient achieved a PR-L (3.3%) for a best response (ORR + PR-L) rate of 96.7% (Table 3). Eight of the 20 patients with PRs met requirements for CR but did not undergo marrow biopsy and were therefore considered PRs per International Workshop on Chronic Lymphocytic Leukemia criteria during the primary study. Treatment-related lymphocytosis was less frequent and less pronounced when ibrutinib was administered in combination with BR than as monotherapy (Figure 1). Only 26.7% of patients treated with the combination experienced an initial treatment-related lymphocytosis. The ORR did not differ significantly based on any clinical features (Figure 2). Hematologic improvement was seen in the majority of patients who were cytopenic at baseline (Table 3).
Best clinical responses in the BR-ibrutinib cohort: primary study (PCYC-1108) and primary study with extension
| Variable . | Primary study (N = 30) . | Primary study and extension period (N = 30) . |
|---|---|---|
| ORR (CR, CRi, nPR, or PR), n (%), (95% CI) | 28 (93.3), (77.9-99.2) | 28 (93.3), (77.9-99.2) |
| ORR + PR with lymphocytosis, n (%), (95% CI) | 29 (96.7), (82.8-99.9) | 29 (96.7), (82.8-99.9) |
| Response categories, n (%) | ||
| CR | 5 (16.7) | 12 (40.0) |
| CRi | 0 (0.0) | 0 (0.0) |
| nPR | 3 (10.0) | 2 (6.7) |
| PR | 20 (66.7) | 14 (46.7) |
| PR with lymphocytosis | 1 (3.3) | 1 (3.3) |
| Progressive disease | 1 (3.3) | 1 (3.3) |
| Sustained hematologic improvement,* n (%) | ||
| Neutropenia (n = 8) | 5 (62.5) | — |
| Anemia (n = 7) | 5 (71.4) | — |
| Thrombocytopenia (n = 14) | 8 (57.1) | — |
| Median duration of response, mo (range)† | Not reached (2.14+ to 16.1+) | Not reached (0.03+ to 38.3+) |
| Estimated rate of duration of response,† (%) | ||
| 6 mo (95% CI) | 88.9 (69.4-96.3) | 92.3 (72.6-98.0) |
| 12 mo (95% CI) | 81.3 (60.8-91.8) | 84.4 (63.7-93.9) |
| 15 mo (95% CI) | 77.2 (56.1-89.1) | — |
| 24 mo (95% CI) | — | 80.4 (59.1-91.4) |
| 36 mo (95% CI) | — | 72.2 (50.2-85.7) |
| 42 mo (95% CI) | — | 72.2 (50.2-85.7) |
| Variable . | Primary study (N = 30) . | Primary study and extension period (N = 30) . |
|---|---|---|
| ORR (CR, CRi, nPR, or PR), n (%), (95% CI) | 28 (93.3), (77.9-99.2) | 28 (93.3), (77.9-99.2) |
| ORR + PR with lymphocytosis, n (%), (95% CI) | 29 (96.7), (82.8-99.9) | 29 (96.7), (82.8-99.9) |
| Response categories, n (%) | ||
| CR | 5 (16.7) | 12 (40.0) |
| CRi | 0 (0.0) | 0 (0.0) |
| nPR | 3 (10.0) | 2 (6.7) |
| PR | 20 (66.7) | 14 (46.7) |
| PR with lymphocytosis | 1 (3.3) | 1 (3.3) |
| Progressive disease | 1 (3.3) | 1 (3.3) |
| Sustained hematologic improvement,* n (%) | ||
| Neutropenia (n = 8) | 5 (62.5) | — |
| Anemia (n = 7) | 5 (71.4) | — |
| Thrombocytopenia (n = 14) | 8 (57.1) | — |
| Median duration of response, mo (range)† | Not reached (2.14+ to 16.1+) | Not reached (0.03+ to 38.3+) |
| Estimated rate of duration of response,† (%) | ||
| 6 mo (95% CI) | 88.9 (69.4-96.3) | 92.3 (72.6-98.0) |
| 12 mo (95% CI) | 81.3 (60.8-91.8) | 84.4 (63.7-93.9) |
| 15 mo (95% CI) | 77.2 (56.1-89.1) | — |
| 24 mo (95% CI) | — | 80.4 (59.1-91.4) |
| 36 mo (95% CI) | — | 72.2 (50.2-85.7) |
| 42 mo (95% CI) | — | 72.2 (50.2-85.7) |
Dashes represent data not obtained. Neutropenia was defined as baseline ANC ≤1500/μL, anemia was defined as baseline hemoglobin ≤11.0 g/dL, and thrombocytopenia was defined as baseline platelet count ≤100 000/μL.
CI, confidence interval.
Hematologic improvement is calculated based on the number of patients with the respective cytopenia at baseline.
N = 28. Duration of response defined as the number of months from first documented PR with lymphocytosis or better to disease progression or death or to date of last adequate disease assessment for subjects who achieved PR or better.
Change in absolute lymphocyte count during primary study (PCYC-1108). Single-agent data taken from Byrd et al.33 R/R, relapsed/refractory.
Change in absolute lymphocyte count during primary study (PCYC-1108). Single-agent data taken from Byrd et al.33 R/R, relapsed/refractory.
Overall response rate by subgroup for patients treated with BR-ibrutinib. Number of responders (n)/total (N) in primary study (PCYC-1108).
Overall response rate by subgroup for patients treated with BR-ibrutinib. Number of responders (n)/total (N) in primary study (PCYC-1108).
Of the 30 patients in the BR-ibrutinib cohort, 6 have progressed or died and 24 were censored without an event at a median follow-up of 15.8 months on the primary study. No Richter transformation was reported during the study conduct. The PFS rates at 6 and 12 months on the primary study were 93.1% and 85.9%, respectively (Figure 3A). One patient treated with BR-ibrutinib whose CLL had del(11q22.3) achieved a CR initially but later progressed, and, at time of relapse, had a C481S mutation not present at baseline.37 When considering follow-up in the extension study, median treatment duration was 35.4 months. The ORR including the extended follow-up was 93.3% (28/30 patients) including 12 (40%) CRs, 2 (6.7%) nPRs, and 14 (46.7%) PRs. The median time to CR was 18.2 months (range: 5.7-36.8 months). One additional patient had a PR-L (3.3%); when PR-L was included, the ORR was 96.7% (29/30 patients) (Table 3). The estimated PFS rates with follow-up in the extension study were 86.3% at 12 months, 78.6% at 18 months and 24 months, and 70.3% at 36 months (Figure 3B).
Kaplan-Meier curve of PFS by investigator assessment, BR-ibrutinib arm. (A) PFS estimate for primary study. (B) PFS estimate for primary study and long-term extension.
Kaplan-Meier curve of PFS by investigator assessment, BR-ibrutinib arm. (A) PFS estimate for primary study. (B) PFS estimate for primary study and long-term extension.
FCR cohort
Three men were enrolled on this patient cohort. Their median age was 56 years (range: 55-58 years) and their median time from diagnosis to study enrollment was 38.9 months. They each had the same single prior therapy, specifically frontline lenalidomide and rituximab on a clinical trial. Two of these patients had bulky disease; however, none had high-risk cytogenetics or baseline cytopenias. The regimen was well tolerated, with all 3 patients able to complete 6 cycles of FCR with planned growth factor support and 1 requiring a dose reduction for cytopenia. One serious AE was observed: an instance of gastritis with associated gastrointestinal bleeding, resulting in hospital admission. Nausea and rash were also observed but were grade 1 to 2.
The ORR was 100%, with 2 of 3 patients achieving CR as a best overall response confirmed by CT scan and bone marrow biopsy after completion of FCR. The patient who initially had nPR based on residual bone marrow disease has since improved his response to CR. All 3 patients were rolled over to a long-term extension study to continue treatment with ibrutinib, and 2 patients were confirmed by local assessment to be minimal residual disease negative (<0.01%) by flow cytometry.
Discussion
The CIT combinations BR and FCR have been a mainstay in the treatment of CLL/SLL.8,9,13,38 However, although CIT has made great strides in improving clinical response in CLL/SLL, it is likely not curative for most patients. A goal of newer agents and combination therapies is to induce a high level of durable objective response while minimizing toxicity. Because single-agent ibrutinib has been shown to induce a high response rate and was well tolerated in previous clinical trials of patients with B-cell malignancies, including CLL/SLL,32-34 we conducted a phase 1b study to determine the safety and efficacy of combining ibrutinib with standard CIT combinations in patients with relapsed/refractory CLL/SLL, with the aim of establishing the proof of concept that ibrutinib can be safely combined with CIT in this patient population.
The primary end point of the study was the incidence of prolonged hematologic toxicity starting in cycle 1; no cases of prolonged neutropenia or thrombocytopenia with onset during cycle 1 were observed in this study. Furthermore, the vast majority (76.2%) of patients with baseline cytopenias who were treated with BR-ibrutinib showed a sustained hematologic improvement with a median follow-up of 15.8 months on the primary study. These findings are consistent with the lack of myelotoxicity of ibrutinib,32-34 suggesting that it is an excellent combination partner for CIT. In fact, no added toxicities were observed beyond what would be expected with BR alone (primarily neutropenia) and no AEs resulting in treatment discontinuation of ibrutinib were observed on the primary study. With median follow-up of 3 years including the extension, only 2 patients discontinued due to AE (other malignancies). The risk of second malignancies in CLL patients is higher at baseline,39 so the relationships to study treatments are unclear.40
The combination of ibrutinib and BR also proved to be efficacious compared to what would be expected with BR treatment alone. The ORR and CR rates observed in the BR-ibrutinib treatment arm of the primary study (ORR: 93.3%; CR: 16.7% increasing to 40% with longer follow-up) were higher than those seen in a previous phase 2 trial of patients with relapsed CLL treated with BR alone (ORR: 59%; CR: 9%).13 In both this study and the prior BR study,13 a median 6 cycles of treatment were administered to patients who had received a median of 2 prior regimens. In this study, in contrast to the study with BR alone, responses to BR-ibrutinib were consistent across all prognostic groups, including patients with del17p. In addition to this high and uniform ORR, a high proportion of patients remained progression-free at 12 months (86.3%), as well as at 24 months (78.6%) and 36 months (70.3%). Median PFS has not been reached with a median follow-up of 37.3 months, which compares favorably with the median event-free survival of 15 months reported in the prior BR study.13
Although enrollment in the FCR-ibrutinib treatment arm was suspended due to the infrequency of relapsed purine analog–naïve patients who were candidates for FCR, all 3 patients in the FCR-ibrutinib treatment arm responded well to treatment, with morphologic CR in the bone marrow and no residual adenopathy by CT scan, and with 2 patients achieving minimal residual disease–negative responses. No major toxicities were observed, although no firm conclusions can be made about the safety of this combination due to the limited experience in only 3 patients. These results provide a compelling rationale for further evaluation of the safety and clinical benefit of ibrutinib in combination with the FCR CIT regimen.
Lymphocytosis, a known pharmacodynamic effect of ibrutinib,41,42 was observed in 78% of patients in the phase 1b/2 study of ibrutinib monotherapy.33 Comparatively, and as one would expect, this study showed a reduced, but not absent, incidence of treatment-related lymphocytosis (26.7%) when ibrutinib was used in combination with BR. Consistent with the single-agent phase 1b/2 study,33 a gradual reduction in absolute lymphocyte count was observed over time. The median time to resolution of lymphocytosis in this study was 2.8 months (range: 0.8-4.6 months), compared to 6.2 months in the phase 1b/2 study.42 The ORR in the phase 1b/2 study of single-agent ibrutinib was 71%,33 with an additional 20% of patients achieving a PR-L (combined ORR + PR-L rate: 91%) with a median 20.9 months follow-up. The 93% ORR observed in the present study (ORR + PR-L rate: 97%) reflects a higher proportion of patients achieving responses with the resolution of lymphocytosis and a higher percentage of CRs (16.7% in primary study and 40% with extension), likely in part due to the additional cytoreductive benefits of CIT. The CR rate may have actually been higher in the primary study if all patients with clinical CR were to have undergone the confirmatory marrow biopsy.
For any individual patient, the long-term benefits of treatment with ibrutinib in combination with BR, which appear to lead to higher CRs compared to BR alone or with ibrutinib alone, will need to be considered together with the overall safety profile, given the potential for additional toxicity that the chemotherapy combination may bring. The safety and efficacy observed in this study suggest that the addition of ibrutinib may substantially enhance the clinical benefit of standard CIT treatments for patients with CLL/SLL. The high ORR and favorable toxicity profile observed in this study provided the impetus for the ongoing phase 3 randomized trial assessing the PFS benefit of combining ibrutinib with BR in relapsed/refractory CLL/SLL (#NCT01611090). This phase 3 trial, which will provide additional data on the risks and benefits of ibrutinib-BR compared to placebo-BR in a larger, international, and more varied clinical population, may provide information to guide patient selection for a particular regimen.
The limited experience but high efficacy and preliminary safety seen with FCR-ibrutinib also warrants further study; this regimen is being evaluated in an ongoing phase 2 study in previously untreated younger patients with CLL (#NCT02251548). As future strategies for the treatment of CLL are developed in the era of novel small molecule–targeted therapies, the combination of ibrutinib with CIT should be considered, given the likely enhanced efficacy seen here without significantly increased toxicity.
Authorship
Contribution: All authors contributed to the study design; A.T., J.R.B., J.C.B., P.M.B., I.W.F., J.A.B., J.W.F., K.R., and S.O. contributed to data collection and interpretation; F.C., D.F.J., and T.G. contributed to data analysis and interpretation; and J.R.B. wrote the manuscript, which was edited by all authors, all of whom gave final approval of the manuscript.
Conflict-of-interest disclosure: J.R.B. has served as a consultant for Pharmacyclics, Janssen, Celgene, Roche, and Genentech. J.A.B., P.M.B., and J.C.B. have served as consultants for Pharmacyclics and received research funding from Pharmacyclics. A.T., F.C., D.F.J., and T.G. are employees and stockholders of Pharmacyclics. S.O. has received research support from Pharmacyclics and Janssen. I.W.F. has served as a consultant and on speakers bureaus for Pharmacyclics and has received research support from Pharmacyclics. J.W.F. and K.R. declare no competing financial interests.
Correspondence: Jennifer R. Brown, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: jennifer_brown@dfci.harvard.edu.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Zeena Salman for trial management and Ying Luan for statistical support. Medical writing assistance was provided by Karen Pemberton and funded by Pharmacyclics.
This study was supported by research funding from Pharmacyclics (Sunnyvale, CA), which provided each site with the pharmaceuticals and funding for the research costs of the study.