In this issue of Blood, Rieger et al show that malaria parasite infiltration in the human placenta requires a specific geometry and affinity of host receptors to facilitate strong adhesion.1
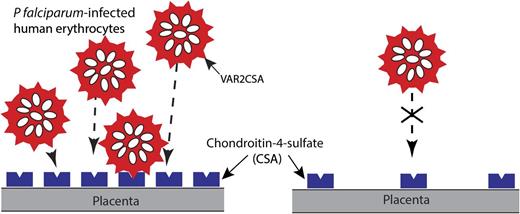
Schematic representation of the adhesion of P falciparum-infected erythrocytes to blood capillaries in the human placenta. A variant of PfEMP1 encoded by the var2CSA gene, expressed on the surface of infected erythrocytes, binds to CSA, a component of the proteoglycan matrix on placenta. PfEMP1 is presented in knob-like protrusions, and sequestration of infected erythrocytes in the placenta is believed to result in maternal malaria, a severe syndrome associated with low birth weight, prematurity, and chronic intervillositis. Higher receptor density (CSA) leads to greater binding and cooperativity as measured by functionalized membranes model system in vitro.1
Schematic representation of the adhesion of P falciparum-infected erythrocytes to blood capillaries in the human placenta. A variant of PfEMP1 encoded by the var2CSA gene, expressed on the surface of infected erythrocytes, binds to CSA, a component of the proteoglycan matrix on placenta. PfEMP1 is presented in knob-like protrusions, and sequestration of infected erythrocytes in the placenta is believed to result in maternal malaria, a severe syndrome associated with low birth weight, prematurity, and chronic intervillositis. Higher receptor density (CSA) leads to greater binding and cooperativity as measured by functionalized membranes model system in vitro.1
Malaria is a prehistoric disease caused by Plasmodium, a genus of parasitic protozoans. Mentioned in the ancient Chinese, Greek, Sanskrit, and Roman literature, the Italian word “mal'aria” for “bad air” refers to an infection that has profoundly influenced the history of human civilization. Early identification of the pigmented protozoa in red blood cells (RBCs) by Charles Laveran in 1880 (Nobel Prize 1907) and of malaria transmission by mosquitoes by Ronald Ross in 1897 (Nobel Prize 1902) set the stage for developing effective remedies to cure and eliminate malaria from the planet. However, the disease remains deadly today, killing an estimated 700 000 to 1 000 000 people each year, particularly in sub-Saharan Africa.2
To combat this global epidemic, there has been great interest in developing new drugs and vaccine(s) against malaria. However, the outcome of these efforts remains unimpressive. Early recognition of the anti-fever properties of bark from the Peruvian cinchona tree, and later transfer of this knowledge by Jesuit priests, led to the identification of quinine and its derivatives as potent antimalarial drugs. In the 1960s, recognition of similar antipyretic effects of qinghaosu from Artemisia annua/sweet wormwood trees led to the discovery of artemisinins by Chinese scientists.3 Combination therapy consisting of artemisinins, quinine derivatives, and supplemental drugs still remains the best, and perhaps the only, practical therapeutic option against malaria. Despite intense research efforts and investment by traditional and philanthropic organizations, no molecularly targeted drug has been developed against malaria, and no effective vaccine exists as yet for the large-scale immunization of affected populations in developing countries. Due to emerging drug resistance against known antimalarial drugs, new therapies and vaccines are urgently needed against this devastating disease.
A key feature of malaria pathogenesis involves the adhesion of malaria parasite-infected RBCs to host endothelial cells. This phenomenon is unique to Plasmodium falciparum, which synthesizes a virulence factor termed PfEMP1 (P falciparum erythrocyte membrane protein-1) during its maturation in host RBCs. Molecular identification of PfEMP1, and its highly polymorphic nature regulated by ∼60 var genes in the parasite genome, constituted a landmark discovery in the malaria field.4-6 PfEMP1 anchors to the host RBC membrane by engaging both host- and parasite-derived cytoskeletal adapters,7 thus exposing its variable ectoplasmic domain to bind to multiple receptors on host endothelial cells. It is believed that firm adhesion of infected RBCs to endothelial receptors contributes to the development of cerebral malaria and other pathologies, including severe complications for both mother and child during pregnancy.
During the blood stage of infection, PfEMP1 interacts with various host receptors, including chondroitin-4-sulfate (CSA), to avoid immune surveillance and splenic clearance. Rieger et al show that it is not only the presence of a receptor that dictates binding, but the receptor-to-receptor distance that also exerts a significant impact on adhesion of infected RBCs (see figure). Using a supported membrane model in which CSA concentration could be modulated, this group shows that, under static conditions, binding of infected RBCs increases with higher CSA density and higher incubation time. Additionally, the cell contact area increased as the distance between CSA molecules decreased in a manner that suggests cooperativity in the binding process. To investigate the role of parasite maturation and CSA binding, trophozoite and schizont detachment was analyzed through the application of ultrasonic pressure waves. Unlike shear stress, this method requires high loading rates as well as all bonds between PfEMP1 and CSA rupture instantaneously and simultaneously for a cell to detach, and, hence, higher unbinding forces. They observed that schizont adherence was higher than that of trophozoites; however, each population followed the same sigmoidal relationship where reduction in the distance between CSA molecules led to increased critical pressure. Under physiological shear stress conditions, they measured the adherence of infected RBCs as a function of CSA distance. Not surprisingly, the smaller the distance between CSA molecules, the higher the number of adherent cells, in a fashion that also suggests cooperative binding. Overall, the new findings have implications for the fundamental binding mechanics of PfEMP1 to CSA and other host receptors. The development of a novel “supported membrane system” used in the context of malaria binding will also be useful for model systems in which the receptor density plays an important role during cellular adhesion pathways.
Although this study rigorously characterizes the conditions affecting the binding parameters of infected RBCs to CSA, it offers limited insights into the translational implications of these findings. This point is relevant because no therapeutic strategies have proven successful in reducing the adhesion of infected RBCs since the discovery of the PfEMP1-mediated adhesion pathway nearly 20 years ago. Perhaps it may be an opportune time to develop cell-permeable strategies aimed at disrupting the interactions of the conserved cytoplasmic segment of PfEMP1 with the cytoskeleton. This approach may potentially mitigate the lesions caused by the cellular adhesion of malaria parasite-infected RBCs to host endothelium.
Conflict-of-interest disclosure: The author declares no competing financial interests.