Key Points
Thrombospondin 1 requires the presence of VWF to modulate arterial thrombosis.
Platelet thrombospondin 1 contributes to arterial thrombosis.
Abstract
Thrombospondin 1 (TSP1) has been suggested as a counter receptor to platelet glycoprotein Ibα that supports initial platelet adhesion in absence of von Willebrand factor (VWF). Conversely, several other studies have shown that TSP1 interacts with VWF and may play a mechanistic role in modulating thrombosis. However, the in vivo evidence to support this mechanism remains unclear. Using intravital microscopy, in a 10% FeCl3-induced thrombosis model, we report similar platelet adhesion in Tsp1-/-/Vwf−/− mice compared with littermate Vwf−/− mice, suggesting that TSP1 does not mediate initial platelet adhesion in the absence of VWF. Tsp1−/− mice exhibited prolonged occlusion time and a significant decrease in the rate of thrombus growth (P < .05 vs wild-type), but not in the initial platelet adhesion. Complete deficiency of VWF abrogated the rate of thrombus growth in Tsp1−/− mice; therefore, we generated Tsp1-/-/Vwf+/− mice to determine whether TSP1 modulates thrombus growth under conditions of partial VWF deficiency. Tsp1-/-/Vwf+/− mice exhibited delayed thrombus growth kinetics and prolonged occlusion time (P < .05 vs Vwf+/−). Finally, we demonstrate that platelet-derived TSP1 modulates arterial thrombosis in vivo. We conclude that TSP1 released from platelets plays a mechanistic role in modulating thrombosis in the presence of VWF.
Introduction
Platelet glycoprotein Ibα (GPIbα) and its ligand von Willebrand factor (VWF) play an important role in mediating platelet adhesion and subsequent thrombus formation at the site of vascular injury.1-5 GPIbα is mainly expressed on platelets and megakaryocytes. VWF, a multimeric glycoprotein, is stored in the Weibel-Palade bodies of endothelial cells and platelet α-granules in the form of ultra-large multimers (ULVWF),6 which are released in circulation upon endothelial cell activation or injury and platelet activation. In comparison with VWF multimers, ULVWF is considered hyperactive because it is more effective in mediating both platelet-vessel wall and platelet-platelet interactions.7,8 Under conditions of high shear, a disintegrin-like and metalloprotease with thrombospondin type I repeats-13 (ADAMTS13), a plasma protease, cleaves ULVWF into smaller and less active VWF multimers.9
For several years the conventional view was that GPIbα binding to VWF on the subendothelial matrix is the essential first step for initial tethering of platelets to the injured vessel wall, particularly at the arterial shear rate.10 Subsequent to initial adhesion a primary hemostatic plug develops at the site of injury by cohesion of platelets through multiple ligand-receptor interactions primarily involving VWF, fibrinogen, and platelet integrin αIIbβ3.11 Mice deficient in VWF,4 and intriguingly, mice deficient for both fibrinogen (Fg) and VWF,2,12 exhibit a decreased but discernible thrombus growth in experimental models of arterial thrombosis suggesting existence of alternative ligands and platelet receptors that can mediate platelet adhesion and subsequent thrombus formation. Markedly increased fibronectin content in platelets of Fg and VWF double-deficient mice suggested that plasma fibronectin (pFN) could support thrombus growth in absence of both Fg and VWF.2 Subsequent follow-up studies using triple-deficient mice for Fg, VWF, and pFN showed that pFN actually inhibited arterial thrombosis in absence of both Fg and VWF.13 Another in vitro study suggested that thrombospondin 1 (TSP1), which is abundantly present in platelet α-granules, may mediate platelet adhesion and subsequent thrombus formation in the absence of VWF.14
TSP1 is a homotrimeric multidomain glycoprotein synthesized by several cell types including fibroblasts,15 smooth muscle cells,16 endothelial cells,17 inflammatory cells,18 and megakaryocytes; it is abundantly stored in platelet α-granules and released upon activation.19 Under arterial shear conditions, immobilized TSP1 has been shown to mediate platelet adhesion through interactions with platelet GPIbα, independent of VWF; thus, it was proposed to be an alternate ligand for GPIbα.14 Recently, another platelet receptor CD36 has been proposed as a counter ligand for TSP1, which mediates platelet adhesion and thrombus growth in mice models of arterial thrombosis.20 On the other hand, TSP1 has been shown to compete with ADAMTS13 for binding A2 and A3 domains of VWF, thus protecting it from proteolytic cleavage.21 TSP1 has also been shown to regulate multimer size of circulating VWF, or VWF released from activated platelets.22,23 Together, these studies suggest that TSP1 may modulate arterial thrombosis via VWF; however, definitive in vivo evidence to support this mechanism remains to be elucidated.
In this study, we generated novel mutants Tsp1-/-/Vwf−/−, Tsp1-/-/Vwf+/− mice and compared them with Tsp1−/−, Vwf−/−, Vwf+/− and wild-type (WT) mice to determine the combined roles of VWF and TSP1 in arterial thrombosis. Our findings reveal that TSP1 does not mediate initial platelet adhesion in the absence of VWF. Interestingly, TSP1 requires VWF to modulate rate of thrombus growth in injured arterioles, suggesting another novel mechanism that regulates thrombosis.
Materials and methods
Animals
Tsp1−/− mice24 (C57BL/6J) and control age-matched WT mice on C57BL/6J background were obtained from The Jackson Laboratory (Bar Harbor, ME). To generate Tsp1−/−/Vwf−/− mice for arterial thrombosis studies, Vwf−/− mice1 (backcrossed >15 times to C57BL/6J) were crossed to Tsp1−/− mice. Tsp1+/+/Vwf−/− and Tsp1−/−/Vwf−/− mice were siblings obtained from crosses of Tsp1+/−/Vwf−/− mice. Tsp1−/−/Vwf+/− were obtained by crossing Tsp1−/−/Vwf−/− to Tsp1−/−/Vwf+/+. Both male and female mice approximately 4 to 5 weeks of age and between 13 to15 g in body weight were used in the study, unless otherwise mentioned. Infused platelets were isolated from 4-to 6-month-old mice of the same genotype. The University of Iowa Animal Care and Use Committee approved all procedures.
Visualization of platelet adhesion and thrombus using intravital microscopy
Platelets from adult donor mice were prepared as described25,26 and briefly explained in the supplemental Methods on the Blood Web site. Intravital microscopy was performed as described.25,26 Platelets labeled with fluorescent calcein green, acetoxymethyl (AM) esters (1.25 × 109 platelets/kg) or fluorescent calcein red-orange, AM were infused through the retro-orbital plexus in mice anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. An incision was made through the abdominal wall to expose the mesentery, and the arterioles of diameter (approximately 100-150 μm) were studied. The exposed mesentery was kept moist and warm by superfusion with warm phosphate saline (37°C). Whatman paper saturated with ferric chloride (10%) solution was applied topically for 5 minutes and thrombus formation in the injured vessel was monitored in real time by using Nikon inverted microscope equipped with 2-channel imaging. Each injured vessel was recorded by using a high-speed EM camera for 40 minutes or until occlusion and movies were evaluated offline using a Nikon computer-assisted image analysis program.
Quantitative analysis of arteriolar thrombus formation
We evaluated (1) the single platelet-vessel wall interactions determined as the number of fluorescent-labeled platelets that deposited on the 250 µm vessel wall segment during 1 minute (3-4 minutes after injury), and platelet counts >150 were counted as 150 for statistical analysis; (2) the time required for formation of thrombus >20 μm in diameter; (3) we quantified thrombus kinetics (growth rate) by following the diameter of ≥30 μm thrombus over a period of 2 minutes, and the fold increase was then calculated by dividing the diameter of thrombus at given time (n) by the diameter of the same thrombus at time (0) . Time 0 is defined as the time point at which the thrombus diameter first reached approximately 30 μm; and (4) occlusion time of the vessel (ie, the time required for blood to stop flowing for 30 seconds).
Platelet depletion and transfusion
Platelet depletion and transfusion in GPIbα/human IL4R transgenic mice were done as described.27 Anti–hIL-4R (clone 25463; R&D Systems) antibody at a concentration of 2.5 μg/g body weight was infused through retro-orbital plexus to deplete platelets from GPIbα/human IL4R transgenic mice. After 2 hours, 5 × 108 platelets from WT or Tsp1−/− mice were injected through retro-orbital plexus, and mice were subjected to FeCl3 injury model as described above.
BM experiments
Bone marrow transplantation (BMT) studies were carried out as described.28 Briefly, recipient Tsp1−/− and WT mice (age 4 weeks) were irradiated with 2 doses of 6.5 Gy at an interval of 4 hours between the first and second irradiations. For transplantation, recipient mice were injected intravenously with 107 bone marrow (BM) cells from pools of BM from either Tsp1−/− or WT mice. All mice were on the C57BL/6J background. After transplantation, mice were maintained in sterile cages and fed autoclaved food and water ad libitum. BMT success was analyzed after 4 weeks by polymerase chain reaction analyses for the WT and Tsp1−/− genomic DNA in peripheral blood mononuclear cells from transplanted mice. Circulating blood cells were counted to ascertain that BMT did not affect the number of BM-derived blood cells.
Statistical analysis
Values are expressed as mean ± standard error of the mean. Significance was analyzed using 1-way analysis of variance followed by Newman-Keuls multiple comparisons test. A value of P < .05 was considered statistically significant.
Results
Tsp1−/− platelets exhibited normal GPIbα expression, integrin αIIbβ3 activation, α-granule release, and platelet aggregation in vitro
We examined whether TSP-1 deficiency affects platelet surface GPIbα expression, integrin αIIbβ3 activation, and granule release by flow cytometry. TSP-1 deficient platelets showed normal GPIbα levels (CD42b) (supplemental Figure 1A). Upon stimulation with thrombin (0.1 U/mL), Tsp1−/− platelets exhibited similar αIIbβ3 integrin activation (JON/A) and P-selectin expression (CD62P) compared with WT platelets (supplemental Figure 1B). To determine if TSP1 deficiency modulates platelet function in vitro, we performed aggregation assay using washed platelets (stimulated with thrombin) and platelet rich plasma (stimulated with adenosine 5′-diphosphate and collagen). No significant difference in platelet aggregation was observed in Tsp1−/− platelets compared with WT platelets (supplemental Figure 2).
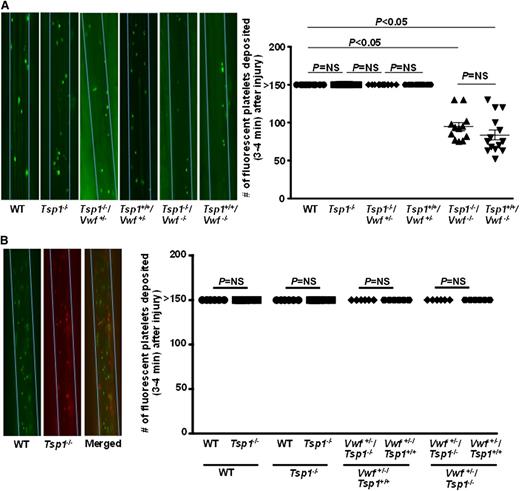
TSP1 deficiency did not significantly alter platelet adhesion to FeCl3-injured arteriolar wall in absence or presence of VWF
Unlike Vwf−/− mice, platelet adhesion at arterial shear conditions is completely inhibited in mice lacking platelet GPIbα, suggesting that other receptors such as GPVI cannot support platelet tethering and adhesion to the injured vessel wall in absence of GPIbα.4 Interestingly, another in vitro study suggested TSP1 may mediate GPIbα-dependent platelet adhesion in the absence of VWF.14 To determine whether TSP1 mediates platelet adhesion via VWF-dependent or independent mechanisms, we compared platelet adhesion in WT, Tsp1−/−, Tsp1+/+/Vwf−/−, and Tsp1-/ -/Vwf−/− mice. We found that the number of fluorescently labeled platelets that adhered transiently to arterioles after FeCl3 injury was similar in Tsp1−/− and WT mice (Figure 1). In concurrence with published reports,1,2 the number of fluorescently labeled platelets that adhered to injured arterioles were significantly less in Vwf−/− mice when compared with WT mice (P < .05) (Figure 1). Initial platelet adhesion to injured arterioles in Tsp1-/ -/Vwf-/- mice was similar to Tsp1+/+/Vwf−/− mice. To determine the effect of VWF heterozygosity (∼50% VWF levels)1 on TSP1-mediated initial platelet adhesion, we compared platelet adhesion in Tsp1+/+/Vwf+/−, Tsp1-/-/Vwf+/−, and WT mice. We found that initial platelet adhesion was similar in Tsp1+/+/Vwf+/−, Tsp1-/-/Vwf+/−, and WT mice (Figure 1A). In another set of experiments, platelets from WT and Tsp1−/− mice were differentially fluorescently labeled and infused in recipient WT or Tsp1−/− mice. After FeCl3 injury, initial platelet adhesion was quantified simultaneously using multichannel intravital microscopy. The same dual platelet labeling protocol was used to quantify single platelet adhesion in Tsp1+/+/Vwf+/− and Tsp1-/-/Vwf+/− mice. Platelet adhesion was found similar in WT, Tsp1−/−, Tsp1+/+/Vwf+/−, and Tsp1-/-/Vwf+/− mice (Figure 1B). Together these findings suggest that TSP1 does not contribute to initial platelet adhesion to the injured vessel wall in the absence or presence of VWF in a 10% FeCl3 injury model.
TSP1 did not significantly contribute to initial platelet adhesion to injured arterioles either in presence or absence of VWF. Using intravital microscopy the number of fluorescent-labeled platelets that deposited on the 250 µm vessel wall segments within 3 to 4 minutes after FeCl3 injury was quantitated. Platelet counts >150 were counted as 150 for statistics. (A) Left panel shows representative photomicrographs of single platelet adhesion (3-4 minutes) after injury. Blue line delineates the vessel. Right panel shows quantification of platelets adhered to the injured mesenteric arteriole after injury. N = 10 to 14 mice/group. (B) Left panel shows representative photomicrographs of adhesion of platelets from WT (calcein green, AM labeled) and Tsp1−/− (calcein red-orange AM labeled) in recipient WT mice using 2-channel imaging. Right panel shows quantification of differential fluorescently labeled platelets that adhered within 3 to 4 minutes after injury. N = 6 mice/group. Data are represented as mean ± standard error of the mean. NS, nonsignificant.
TSP1 did not significantly contribute to initial platelet adhesion to injured arterioles either in presence or absence of VWF. Using intravital microscopy the number of fluorescent-labeled platelets that deposited on the 250 µm vessel wall segments within 3 to 4 minutes after FeCl3 injury was quantitated. Platelet counts >150 were counted as 150 for statistics. (A) Left panel shows representative photomicrographs of single platelet adhesion (3-4 minutes) after injury. Blue line delineates the vessel. Right panel shows quantification of platelets adhered to the injured mesenteric arteriole after injury. N = 10 to 14 mice/group. (B) Left panel shows representative photomicrographs of adhesion of platelets from WT (calcein green, AM labeled) and Tsp1−/− (calcein red-orange AM labeled) in recipient WT mice using 2-channel imaging. Right panel shows quantification of differential fluorescently labeled platelets that adhered within 3 to 4 minutes after injury. N = 6 mice/group. Data are represented as mean ± standard error of the mean. NS, nonsignificant.
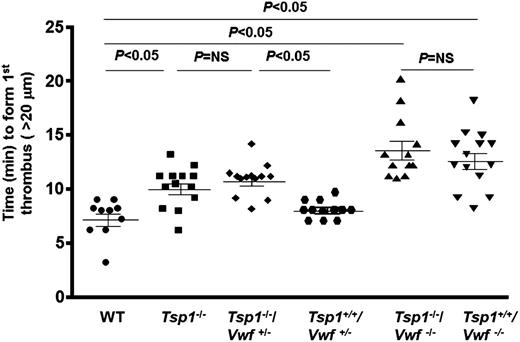
TSP1 requires VWF to modulate initial thrombus formation
To determine whether TSP1 modulates initial thrombus formation via VWF-dependent or independent mechanisms, we measured the time to form first thrombus (>20 μm) in the injured arterioles of WT, Tsp1−/−, Tsp1-/-/Vwf+/−, Tsp1+/+/Vwf+/−, Tsp1-/-/Vwf−/−, and Tsp1+/+/Vwf−/−mice. The mean time to form first thrombus was significantly increased in Tsp1−/− mice compared with WT (9.98 ± 0.52 minutes vs 7.12 ± 0.55 minutes; P < .05) (Figure 2). As expected, and similar to previous published reports,1,2 Vwf−/− mice exhibited a significant delay in time to form first thrombus compared with WT mice (13.57 ± 0.85 minutes vs 7.12 ± 0.55 minutes; P < .05) (Figure 2). The mean time to form first thrombus was similar in Tsp1−/−/Vwf−/−, and Tsp1+/+/Vwf−/− mice (Figure 2), suggesting that TSP1 requires the presence of VWF to modulate initial thrombus formation. We generated Tsp1-/-/Vwf+/− mice to determine whether TSP1 modulates thrombus growth under conditions of partial VWF deficiency (∼50% VWF levels). The mean time to form first thrombus was significantly delayed in Tsp1−/−/Vwf+/− when compared with Tsp1+/+/Vwf+/− mice (10.37 ± 0.51 minutes vs 8.07 ± 0.25 minutes; P < .05) (Figure 2). Taken together, these findings suggest that TSP1 requires VWF to modulate initial thrombus formation.
TSP1 deficiency significantly delays time to form first thrombus in presence of VWF. Using intravital microscopy time to first thrombus (>20 μm) was quantitated after 10% FeCl3 injury. Dot plot represents the time required to form first thrombus, which was significantly delayed in Tsp1−/− mice when compared with WT mice and the process was VWF-dependent. N = 10 to 14 mice/group. NS, nonsignificant.
TSP1 deficiency significantly delays time to form first thrombus in presence of VWF. Using intravital microscopy time to first thrombus (>20 μm) was quantitated after 10% FeCl3 injury. Dot plot represents the time required to form first thrombus, which was significantly delayed in Tsp1−/− mice when compared with WT mice and the process was VWF-dependent. N = 10 to 14 mice/group. NS, nonsignificant.
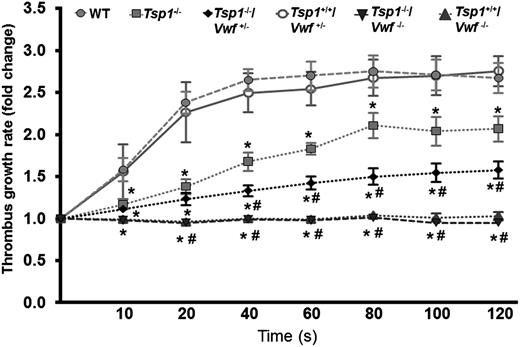
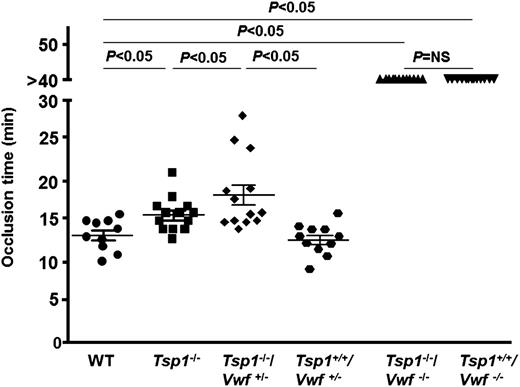
VWF is required for TSP1 to modulate thrombus growth
Next, we determined whether TSP1 modulates later stages of thrombus growth through VWF. We measured thrombus growth kinetics and occlusion time in the injured arterioles. The rate of thrombus growth was significantly decreased in Tsp1−/− mice when compared with WT mice (P < .05) (Figure 3), and the thrombus growth was completely abrogated in both Tsp1+/+/Vwf−/− and Tsp1−/−/Vwf−/− mice (P < .05 vs WT mice) (Figure 3). Because complete deficiency of VWF abrogated thrombus growth and VWF was required to see the effect of TSP1 on initial thrombus formation, we used Tsp1-/-/Vwf+/− mice to determine whether TSP1 modulate later stages of thrombus growth under conditions of partial VWF deficiency. The rate of thrombus growth was significantly decreased in Tsp1-/-/Vwf+/− compared with Tsp1+/+/Vwf+/− mice (P < .05) (Figure 3). Together, these results suggest that the presence of VWF in plasma is required for TSP1 to modulate later stages of thrombus growth. Next, we measured the occlusion time in the injured arterioles. The mean time to complete occlusion was modestly, but significantly, prolonged in Tsp1−/− mice compared with WT mice (15.80 ± 0.59 minutes vs 13.31 ± 0.63 minutes; P < .05) (Figure 4). None of the injured arterioles occluded in Tsp1−/−/Vwf−/− and Tsp1+/+/Vwf−/− mice during the 40 minutes observation time (Figure 4). Compared with Tsp1+/+Vwf+/− mice, the mean occlusion time was markedly prolonged in Tsp1−/−/Vwf+/− (12.70 ± 0.47 minutes vs 18.29 ± 1.26 minutes; P < .05) (Figure 4), confirming once again that VWF is required for TSP1 to modulate thrombus growth. Factor VIII levels in Vwf+/− mice are ∼60% of those in WT mice.1 To rule out the possibility that significant delay in thrombus growth in Tsp1−/−/Vwf+/− mice is not due to factor VIII deficiency, we compared thrombus growth in Vwf+/− and WT mice. The mean time to form first thrombus, thrombus growth kinetics, and time to complete occlusion were similar in WT and Vwf+/− mice, suggesting that the effect of TSP1 on thrombus growth is not due to reduction in factor VIII levels.
TSP1 requires VWF to modulate arterial thrombus growth. The rate of thrombus growth in Tsp1−/− mice was significantly decreased when compared with WT mice (*P < .05). Deficiency of VWF completely abrogated thrombus growth. The rate of thrombus growth was significantly decreased in Tsp1-/-/Vwf+/− compared with Tsp1+/+/Vwf+/− mice (P < .05), suggesting that ∼50% of VWF level in plasma is required for TSP1 to modulate later stages of thrombus growth. Of note Tsp1−/−/Vwf+/− mice exhibited markedly decreased rate of thrombus growth (#P < .05 vs Tsp1−/−). Data represent mean ± standard error of the mean. N = 10 to 13 mice/group. *P < .05 vs WT; #P < .05 vs Tsp1−/−.
TSP1 requires VWF to modulate arterial thrombus growth. The rate of thrombus growth in Tsp1−/− mice was significantly decreased when compared with WT mice (*P < .05). Deficiency of VWF completely abrogated thrombus growth. The rate of thrombus growth was significantly decreased in Tsp1-/-/Vwf+/− compared with Tsp1+/+/Vwf+/− mice (P < .05), suggesting that ∼50% of VWF level in plasma is required for TSP1 to modulate later stages of thrombus growth. Of note Tsp1−/−/Vwf+/− mice exhibited markedly decreased rate of thrombus growth (#P < .05 vs Tsp1−/−). Data represent mean ± standard error of the mean. N = 10 to 13 mice/group. *P < .05 vs WT; #P < .05 vs Tsp1−/−.
TSP1 deficiency in mice prolongs mean time to complete occlusion in injured arteriole. Dot plot represents the time required for complete occlusion, which was quantitated as time required for blood to stop flowing for 30 seconds in arterioles injured with 10% FeCl3. The mean time to complete occlusion was significantly prolonged in Tsp1−/− mice when compared with WT (P < .05). None of the injured vessels occluded in Tsp1−/−/Vwf−/− and Tsp1+/+/Vwf−/−. The mean time to complete occlusion was significantly prolonged in Tsp1−/−/Vwf+/− when compared with Tsp1+/+/Vwf+/−, suggesting a threshold of VWF is required for TSP1 to modulate arterial thrombosis. Data represent mean ± standard error of the mean. N = 10 to 13 mice/group. NS, nonsignificant.
TSP1 deficiency in mice prolongs mean time to complete occlusion in injured arteriole. Dot plot represents the time required for complete occlusion, which was quantitated as time required for blood to stop flowing for 30 seconds in arterioles injured with 10% FeCl3. The mean time to complete occlusion was significantly prolonged in Tsp1−/− mice when compared with WT (P < .05). None of the injured vessels occluded in Tsp1−/−/Vwf−/− and Tsp1+/+/Vwf−/−. The mean time to complete occlusion was significantly prolonged in Tsp1−/−/Vwf+/− when compared with Tsp1+/+/Vwf+/−, suggesting a threshold of VWF is required for TSP1 to modulate arterial thrombosis. Data represent mean ± standard error of the mean. N = 10 to 13 mice/group. NS, nonsignificant.
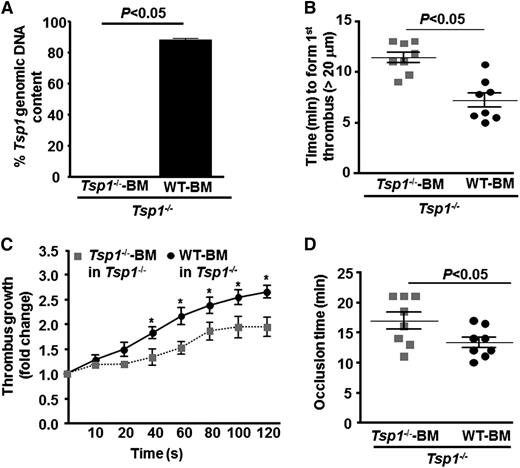
Hematopoietic cell-derived TSP1 contributes to arterial thrombosis
Several cell types synthesize TSP1 including endothelial cells, vascular smooth cells, and hematopoietic-derived cells.15-19 To determine the source of TSP1 that contributes to arterial thrombosis, we transplanted irradiated Tsp1−/− mice with BM from either Tsp1−/− or WT mice. This adoptive transfer resulted in chimeric mice that had normal TSP1 in hematopoietic-derived cells, but lack TSP1 in nonhematopoietic cells including endothelial cells and vascular smooth muscle cells. Four weeks after BMT, >85% of circulating white blood cells from WT-BM→ Tsp1−/− mice had Tsp1 in genomic DNA, whereas 100% of white blood cells from Tsp1−/−-BM→ Tsp1−/− mice lacked Tsp1 (Figure 5A). The total blood cell counts were similar in WT-BM→ Tsp1−/− and Tsp1−/−-BM→ Tsp1−/− mice (not shown). Time to form first thrombus (>20 μm) and mean occlusion time were significantly prolonged in Tsp1−/−-BM→ Tsp1−/− mice when compared with WT-BM→ Tsp1−/− mice (P < .05) (Figure 5 B-D). Similarly, the rate of thrombus growth was significantly decreased in Tsp1−/−-BM→ Tsp1−/− mice when compared with WT-BM→ Tsp1−/− mice (P < .05) (Figure 5C). Next, we confirmed these findings by transplanting irradiated WT mice with BM from either WT or Tsp1−/− mice. Four weeks after BMT, 100% of circulating white blood cells from WT BM→ WT mice contained Tsp1 in genomic DNA, whereas ∼90% of white blood cells from Tsp1−/−-BM→ WT mice lacked Tsp1 (supplemental Figure 3). The total blood cell counts were similar in WT-BM→ WT and Tsp1−/−-BM→ WT mice (not shown). Time to form first thrombus (>20 μm) and mean occlusion time were significantly prolonged, and the rate of thrombus growth was significantly decreased in Tsp1−/−-BM→ WT mice when compared with WT BM→ WT mice (P < .05) (supplemental Figure 3). Together these findings suggest that hematopoietic cell-derived TSP1, but not endothelial-derived TSP1, determines the rate of thrombus growth and modulate arterial thrombosis.
Hematopoietic cell-derived TSP1 modulates arterial thrombosis. (A) Genomic DNA PCR analysis for the Tsp1 gene in peripheral blood mononuclear cells from transplanted Tsp1−/−-BM→ Tsp1−/− mice and WT-BM→ Tsp1−/− mice. (B) Quantification of first thrombus (>20 μm). (C) Thrombus growth kinetics (fold increase). (D) Mean time to complete occlusion. Data represent mean ± standard error of the mean. N = 7 to 8 mice/group. *P < .05; WT-BM in Tsp1−/− vs Tsp1−/−-BM in Tsp1−/− mice.
Hematopoietic cell-derived TSP1 modulates arterial thrombosis. (A) Genomic DNA PCR analysis for the Tsp1 gene in peripheral blood mononuclear cells from transplanted Tsp1−/−-BM→ Tsp1−/− mice and WT-BM→ Tsp1−/− mice. (B) Quantification of first thrombus (>20 μm). (C) Thrombus growth kinetics (fold increase). (D) Mean time to complete occlusion. Data represent mean ± standard error of the mean. N = 7 to 8 mice/group. *P < .05; WT-BM in Tsp1−/− vs Tsp1−/−-BM in Tsp1−/− mice.
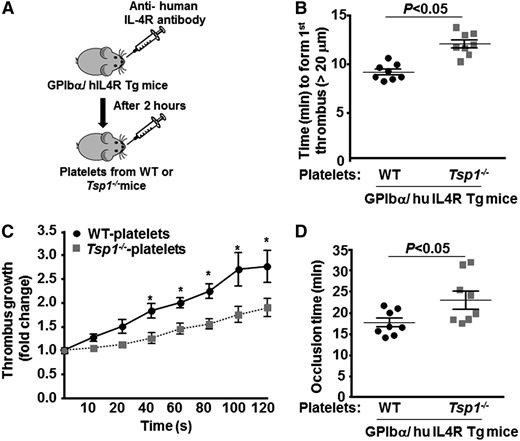
Platelet-derived TSP1 contributes to arterial thrombosis
To specifically determine whether TSP1 released from platelets contributes to arterial thrombosis in vivo, we depleted platelets from GPIbα/human IL4R transgenic mice using anti-hIL4R antibody as described,27,29 followed by transfusion of platelets from either WT or Tsp1−/− mice (Figure 6A). GPIbα/human IL4R transgenic mice lack murine GPIbα, but they express the extracellular domain of human IL-4 receptor (fused to the transmembrane and cytoplasmic domains of human GPIbα). GPIbα/human IL4R transgenic mice lacking platelet TSP1 showed significantly prolonged time to first thrombus formation and complete occlusion, and a decrease in thrombus growth rate when compared with GPIbα/human IL-4 receptor transgenic mice containing platelets with TSP1 (P < .05) (Figure 6 B-D).
Platelet TSP1 contributes to thrombosis in injured arteries. (A) Schematic depicting the technique for generating mice with platelet-specific TSP1 deficiency. (B-D) Graphs representing (B) time to first thrombus formation (C), thrombus growth, and (D) mean occlusion time in FeCl3-injured carotid arteries. Data are presented as mean ± standard error of the mean. N = 7 to 8 mice/group. *P < .05 compared with GPIbα/human IL4R transgenic mice reinfused with TSP1 deficient platelets.
Platelet TSP1 contributes to thrombosis in injured arteries. (A) Schematic depicting the technique for generating mice with platelet-specific TSP1 deficiency. (B-D) Graphs representing (B) time to first thrombus formation (C), thrombus growth, and (D) mean occlusion time in FeCl3-injured carotid arteries. Data are presented as mean ± standard error of the mean. N = 7 to 8 mice/group. *P < .05 compared with GPIbα/human IL4R transgenic mice reinfused with TSP1 deficient platelets.
Discussion
Several past studies have shown that TSP1 interacts with VWF, which may play a mechanistic role in thrombosis.22,23,30-32 However, in vivo evidence to support this mechanism remains unclear and questionable. This prompted us to investigate the role of TSP1 in arterial thrombosis in the context of VWF deficiency. Using a genetic approach in mice, we demonstrate that TSP1 requires ∼50% VWF levels to modulate arterial thrombus growth after 10% FeCl3 injury in mesenteric arterioles, but it does not mediate initial platelet adhesion. In addition, we demonstrate that platelet-derived TSP1, but not endothelial-derived TSP1, modulates arterial thrombosis in vivo.
The multidomain structure of TSP1 allows it to interact with many proteins including collagen, VWF, fibrinogen, and fibronectin, all of which have been shown to be mediating platelet recruitment to subendothelial matrix after injury to the arterial wall,31,33-38 suggesting that TSP1 may contribute to initial platelet adhesion. However, the evidence to support the role of TSP1 in platelet adhesion to the injured vessel wall is not well elucidated and remains debatable. Previous studies under static or flow conditions have shown that TSP1 has both adhesive and antiadhesive properties, depending on the presence or absence of Ca2+ ions, respectively.30,39,40 Agbanyo et al,30 suggested that adhesive function of TSP1 depended on its conformation (requires Ca2+ ions) and is maximal at higher arterial shear rate. These authors showed that platelet adhesion to TSP1 was dependent on α2β1 and αIIbβ3 under certain experimental conditions in vitro. Conversely, another follow-up study by Jurk et al,14 showed that in the absence of VWF, immobilized TSP1 could mediate GPIbα-dependent platelet adhesion at arterial shear conditions. Furthermore, the authors showed that other TSP1 counter receptors including integrins α2β1, αvβ3, αIIbβ3, CD36, CD47, and heparan sulfate do not contribute to platelet adhesion to TSP1 at arterial shear rates. Based on these in vitro findings, the authors proposed that TSP1 acts as an alternate ligand to VWF that may mediate GPIbα-dependent platelet adhesion.14 In contrast to these reports, we found that TSP1 does not mediate initial platelet adhesion to the injured vessel wall under arterial shear conditions in the presence or absence of VWF in vivo. Our results are in agreement with another study that showed only a weak interaction of platelets to the immobilized TSP1 at arterial shear rates.32
Although TSP1 deficiency in mice did not affect initial platelet adhesion, we found that the time to form first thrombus and complete occlusion were significantly prolonged in Tsp1−/− mice compared with WT mice. In addition, we found that TSP1 deficiency in mice significantly decreases thrombus growth kinetics, suggesting that TSP1 determines the rate of thrombus growth after injury under arterial shear conditions. Our findings are in accordance with a previous study that showed TSP1 deficiency in mice resulted in prolonged occlusion time in a photochemical injury-induced mesenteric artery thrombosis model.41 There are several potential mechanisms that have been suggested by which TSP1 could contribute to arterial thrombosis. First, the observation that impaired arterial thrombosis in TSP1-deficient mice could be reversed by infusing neutralizing antibodies against ADAMTS1341 suggests that the role of TSP1 in arterial thrombosis is dependent on its ability to prevent proteolytic cleavage of VWF by ADAMTS13. Indeed, in vitro studies suggested that TSP1 and recombinant ADAMTS13 compete for binding to recombinant VWF A2 and A3 domain.21,22 On the other hand, TSP1 has also been suggested to act as a reductase that controls VWF multimer size, and thereby may negatively regulate thrombus growth.22,23 Second, TSP1 could directly interact with its counter receptors on platelets, particularly CD36,20 to mediate platelet adhesion under arterial shear and thereby promote thrombosis. Although these studies suggest VWF dependent and independent roles for TSP1 in arterial thrombosis, definitive in vivo evidence for either was still lacking. Herein, we demonstrate that TSP1 deficiency on Vwf−/− background had no significant effect on time to form first thrombus, thrombus growth kinetics, and occlusion time. Because the deficiency of VWF completely abrogated thrombus growth in Tsp1-/- mice, we speculated that TSP1 may require some VWF levels in circulation to modulate arterial thrombosis. Indeed, Tsp1-/-/Vwf+/− mice exhibited significant delay in thrombogenesis when compared with Tsp1+/+/Vwf+/− mice, suggesting that TSP1 requires partial VWF levels to modulate arterial thrombosis. The observed decrease in thrombogenesis in Tsp1-/-/Vwf+/− mice was not dependent on VWF-mediated platelet recruitment or a decrease in factor VIII levels because Vwf+/− mice, which have ∼50% VWF antigen and ∼60% factor VIII levels,1 did not exhibit any defect in initial platelet adhesion, thrombus growth rate, and time to complete occlusion under similar experimental conditions. Of note, the influence of TSP1 deficiency on the rate of thrombus growth was much more pronounced in Vwf+/− mice when compared with TSP1 deficiency in WT mice, suggesting a prominent role for TSP1 in arterial thrombosis under conditions of partial VWF deficiency.
The concentration of TSP1 at the baseline in the plasma ranges from 60 to 80 ng/mL (∼400-500 pM based on molecular weight of 165 kDa)23,42,43 and apparent dissociation constant (Kd) for soluble TSP1 binding to VWF A3 domain is ∼3 μM.22 The Kd for soluble TSP1 binding to VWF A2 domain is not known, but we speculate that it could be similar (∼3 μM) to A3 domain because in the residual collagen binding assay it was shown that TSP1 (100 nM) competes with both VWF A2 and A3 domain to a similar extent.21 On the other hand, the levels of ADAMTS13 in plasma is ∼1000 ng/mL (∼7 nM),44 and has much higher affinity for VWF (Kd ∼ 15 nM).45 Together, these observations suggest that it is highly unlikely that low TSP1 (∼400-500 pM) present at basal levels in plasma could compete with ADAMTS13 (∼7 nM) for VWF A2 and A3 binding. However, platelets have large stores of TSP1 in their α-granules, which are released upon stimulation by strong agonists such as collagen yielding ∼1000-fold increase in plasma TSP-1 levels to 10 to 20 µg/mL (∼100 nM).43,46 At these concentrations, TSP1 has been shown to cause significant (∼50%) inhibition of ADAMTS13 activity.21 Based on these reports, we hypothesized that higher concentrations of TSP1 released from activated platelets within thrombus milieu modulate thrombus growth by preventing cleavage of VWF by ADAMTS13. To test this hypothesis, we depleted platelets in GPIbα-hIL4R transgenic mice and reinfused them with either Tsp1−/− or WT platelets. GPIbα-hIL4R transgenic mice with Tsp1−/− platelets showed significant impairment in thrombus formation and growth compared with those with WT platelets confirming that it is indeed platelet-derived TSP1, which modulates arterial thrombosis in the presence of VWF. This could explain our observations that TSP1 deficiency alters thrombus formation and growth, but not initial platelet adhesion to injured arterial wall.
In summary, we have demonstrated that platelet-derived TSP1 has an essential role in arterial thrombosis that is dependent on the availability of VWF. It remains to be determined whether TSP1 impact is of particular significance in the context of partial VWF deficiency as it occurs in type I von Willebrand disease.47
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chintan Gandhi for helping with the bone marrow transplantation technique. This work was supported by grants from the National Scientist Development (7520015) (A.K.C.), the American Heart Association, and the National Institutes of Health, National Heart, Lung and Blood Institute (R01 HL118246) and (R01 HL118742).
Authorship
Contribution: P.P. and P.P.K. performed experiments and analyzed results; P.P., P.P.K., and A.K.C. interpreted results and cowrote the manuscript; and A.K.C. directed the project and designed the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anil K. Chauhan, University of Iowa, Department of Internal Medicine, 25 S Grand Ave, 3160 Medical Labs, Iowa City, Iowa 52242; e-mail: anil-chauhan@uiowa.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal