Key Points
Administration of plasma to a factor V–deficient individual yields a stable platelet factor V/Va pool derived from megakaryocyte endocytosis.
Platelets and platelet-derived factor V/Va promote and extend hemostasis well after depletion of the plasma-derived factor V pool.
Abstract
Whole genome sequencing of an individual completely devoid of plasma- and platelet-derived factor V (FV) identified 167 variants in his F5 gene including previously identified and damaging missense mutations at rs6027 and Leu90Ser. Because the administration of fresh frozen plasma (FFP) prevents gastrointestinal bleeding in this individual, its effects on his plasma- and platelet-derived FV concentrations were assessed. The patient’s plasma FV levels peaked by 2 hours following FFP administration and were undetectable 96 hours later. In contrast, increased platelet-derived FV/Va concentrations were observed within 6 hours, peaked at 24 hours, decreased slowly over 7 days, and originated from megakaryocyte endocytosis and intracellular processing of plasma FV. Ten days after transfusion, no thrombin was generated in a tissue factor–initiated whole blood clotting assay unless exogenous FV was added, consistent with the complete absence of plasma FV. In marked contrast, release of the patient’s platelet-derived FV/Va (7% of normal) following platelet activation resulted in robust thrombin generation, similar to that in an individual with normal plasma- and platelet-derived FV concentrations. Thus, total FV deficiency can be corrected by plasma administration, which partially repletes and sustains the platelet cofactor pool, thereby highlighting the critical role of platelet-derived FV/Va in ensuring hemostatic competence.
Introduction
Congenital factor V (FV) deficiency is a rare autosomal recessive bleeding disorder (prevalence ∼1:1 000 000).1 Individuals heterozygous for this disorder are usually asymptomatic.1,2 However, the bleeding phenotype in individuals with undetectable levels of FV antigen and activity (<1%) in their plasma varies dramatically.1,2 Although the majority of the total FV pool circulates in plasma, ∼20% to 25% is stored in platelet α-granules (4600-14 000 molecules per platelet).3 This platelet-derived FV pool originates solely from megakaryocyte endocytosis of the plasma procofactor through a process that results in the formation of a partially proteolytically activated cofactor (FV/Va)4 and phenotypically alters it to a more procoagulant phenotype.4-10
The most common treatment of individuals with symptomatic FV deficiency is administration of fresh frozen plasma (FFP) to temporarily maintain plasma FV at minimally hemostatic levels (20% to 30%).11 Its effect on platelet-derived FV/Va concentrations is unknown. In the current investigation, an individual with undetectable levels of both plasma- and platelet-derived FV/Va,3 who receives fresh frozen plasma (FFP) transfusions to control gastrointestinal (GI) bleeding, was studied.
Study design
Patient history
A 67-year-old man with congenital FV deficiency (<1% plasma- and platelet-derived FV antigen and activity)3 was recruited and consented according to a protocol approved by the University of Vermont Committee on Human Research. The patient experienced recurrent epistaxis and prolonged bleeding after dental surgery and underwent left hip hemiarthroplasty and right knee total arthroplasty for end-stage arthropathy caused by recurrent hemarthroses. Both surgeries required subsequent revision. When studied initially (February 2005), the patient was receiving 2 units of FFP per week to prevent GI bleeding. At follow-up (August 2008 and October 2012), he only required 2 units of FFP every 2 weeks.
Whole genome sequencing
Whole genome sequencing and subsequent analyses were performed by the University of Vermont Advanced Genome Technologies Core Facility. DNA, isolated from peripheral blood, was sequenced on an Illumina HiSequation 1000 sequencer (average of 25 ± 5 reads). The data were analyzed using the Genome Analysis Toolkit. Variants were evaluated for biological relevance with Polymorphism Phenotyping v2 and Scale-invariant feature transform algorithms.
Assessment of plasma-derived FV antigen and activity
FV antigen was determined by a competitive radioimmunoassay.3 Plasma-derived FV levels between 0 and 2 hours of FFP administration were extrapolated based on the FV turnover rate in a nonhuman primate model12 assuming a starting plasma volume of 3200 mL (hematocrit = 36%) and a constant transfusion rate (3.75 mL plasma/min).
Western blotting analyses of platelet- and plasma-derived FV
Plasma and platelet-derived FV/Va was visualized by western blotting as detailed previously.9 For quantitative western blotting analyses, platelet lysates5 were treated with thrombin (2 U/mL, 10 minutes, 37°C) to convert all platelet-derived FV/Va to FVa. The density of the platelet-derived FVa heavy and light chains was compared with a standard curve prepared from an unaffected control presumed to have ∼10 0003 molecules FV per platelet.
Whole blood coagulation
Tissue factor (TF)–initiated whole blood clotting assays and quantification of serum thrombin-antithrombin complex (TAT) formation were performed as described.13 Whole blood clotting reactions contained: (1) TF (5 pM) and corn trypsin inhibitor (CTI) (100 µg/mL); (2) TF, CTI, and FV (2 nM); (3) TF, CTI, and protease activated receptor (PAR) 1 (100 μM) and PAR4 (500 μM) agonist peptides; and (4) CTI alone.
Measurement of TFPI
Plasma tissue factor pathway inhibitor (TFPI) was quantified using Quantikine Human TFPI Immunoassay (R&D Systems, Minneapolis MN).
Results and discussion
Whole genome sequencing identified 167 variants in the patient’s F5 gene. Two variants, rs6027 (A6755G mutation causing an Asp2194Gly substitution in the FV C2 domain) and L90S (an A to G mutation at chr1:169541563 causing a Leu90Ser [Leu62Ser] substitution in the FV A1 domain), were classified as damaging, having been shown previously to be associated with FV deficiency.14,15 The patient is heterozygous at both loci, which may explain his complete absence of FV; however, other variants may play a role.
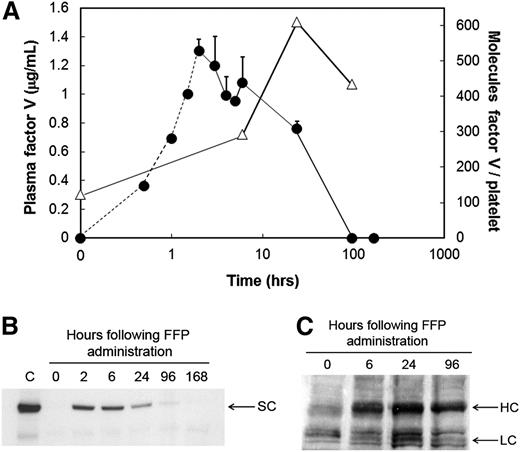
Following FFP administration, the patient’s plasma FV concentration increased from undetectable (t = 0 hours) to 1.3 µg/mL (2 hours) (Figure 1A, circles), declined rapidly, and was undetectable by 96 hours. These data were confirmed by western blotting (Figure 1B). In contrast, quantifiable levels of platelet-derived FV/Va were observed prior to FFP administration (∼124 molecules per platelet) (Figure 1A, triangles; Figure 1C), which presumably represented platelet-derived FV/Va remaining from the previous transfusion. Platelet-derived FV/Va nearly doubled by 6 hours, peaked at 24 hours post–FFP administration (609 molecules per platelet) (Figure 1A, triangles), with a substantial amount remaining (434 molecules per platelet) 96 hours posttransfusion (Figure 1A, triangles). A follow-up study confirmed that the rapid acquisition of FV by the patient’s platelets was the result of megakaryocyte and not platelet endocytosis of the plasma molecule (supplemental Figure 1; available on the Blood Web site), and that following endocytosis, the patient’s platelet-derived FV/Va was proteolytically processed normally4 (supplemental Figure 2).
Quantification of plasma- and platelet-derived FV levels in an FV-deficient patient prior to and subsequent to administration of FFP. (A) Plasma-derived FV antigen and activity was measured prior to (time = 0 hours) and subsequent to FFP administration, at the times indicated, using a double antibody competitive radioimmunoassay (circles). The FV concentrations between 0 and 2 hours (circles, dashed line) were extrapolated based on the human FV turnover rate in a nonhuman primate model as described in “Study design.” Platelet-derived FV (triangles) was measured using a quantitative western blot described in “Study design” using washed platelets lysed with triton X-100 in the presence of leupeptin. Platelet lysates were treated with thrombin (2 U/mL, 10 minutes, 37°C) to convert all platelet-derived FV and its partial activation products to FVa. Following sodium dodecyl sulfate–polyacrylamide gel electrophoresis, immunoblotting was performed using a mixture of an anti-FV heavy chain and an anti-FV light chain monoclonal antibody. (B) FV in plasma was immunoblotted prior to (0 hours) and subsequent to FFP administration (6, 24, 96, and 168 hours) as described above. The position of single chain FV (SC) is indicated. C, plasma from an unaffected individual. (C) Platelet-derived FV was immunoblotted in whole platelet lysates following its conversion to FVa prior to (0 hours) and subsequent to FFP administration (6, 24, and 96 hours), as described above. The positions of the FVa heavy chain (HC) and light chain (LC) are indicated.
Quantification of plasma- and platelet-derived FV levels in an FV-deficient patient prior to and subsequent to administration of FFP. (A) Plasma-derived FV antigen and activity was measured prior to (time = 0 hours) and subsequent to FFP administration, at the times indicated, using a double antibody competitive radioimmunoassay (circles). The FV concentrations between 0 and 2 hours (circles, dashed line) were extrapolated based on the human FV turnover rate in a nonhuman primate model as described in “Study design.” Platelet-derived FV (triangles) was measured using a quantitative western blot described in “Study design” using washed platelets lysed with triton X-100 in the presence of leupeptin. Platelet lysates were treated with thrombin (2 U/mL, 10 minutes, 37°C) to convert all platelet-derived FV and its partial activation products to FVa. Following sodium dodecyl sulfate–polyacrylamide gel electrophoresis, immunoblotting was performed using a mixture of an anti-FV heavy chain and an anti-FV light chain monoclonal antibody. (B) FV in plasma was immunoblotted prior to (0 hours) and subsequent to FFP administration (6, 24, 96, and 168 hours) as described above. The position of single chain FV (SC) is indicated. C, plasma from an unaffected individual. (C) Platelet-derived FV was immunoblotted in whole platelet lysates following its conversion to FVa prior to (0 hours) and subsequent to FFP administration (6, 24, and 96 hours), as described above. The positions of the FVa heavy chain (HC) and light chain (LC) are indicated.
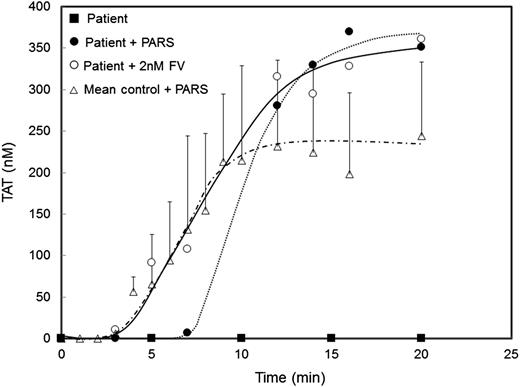
The ability of the patient’s platelets and platelet-derived FV/Va to support thrombin generation 10 days after FFP administration was assessed in a TF-dependent, contact pathway-suppressed, whole blood clotting assay13 following platelet activation with PAR1 and PAR4 agonist peptides (supplemental Figure 3). Simultaneous addition of TF and the agonist peptides had little effect on whole blood clotting and thrombin formation in an unaffected individual (supplemental Figure 4). Although no thrombin was generated in the absence of added FV (Figure 2, closed squares), the simultaneous addition of PAR1 and PAR4 agonist peptides to the patient’s blood resulted in platelet clumping by 3.8 minutes and clot formation by 7.8 minutes. Thrombin generation was robust (37.5 nM thrombin/minute) with a maximum level of thrombin equal to 369.4 nM (Figure 2, closed circles). In comparison, FV addition (2 nM) to the patient’s blood shortened the clot time (∼2.8 minutes) but effected thrombin generation at a nearly identical rate (40.6 nM thrombin/min) and amplitude (360.9 nM thrombin) (Figure 2, open circles). When the effects of the agonist peptides on the whole blood clotting profiles of the patient (Figure 2, closed circles) and 2 unaffected individuals (Figure 2, triangles) were compared, only the durations of the initiation phases were substantially different (∼7.8 minutes vs ∼2.8 ± 0.35 minutes).
The patient’s platelet-derived FV pool remaining 10 days after plasma administration supports thrombin generation in a TF-initiated whole blood clotting model. Whole blood from the patient was incubated (at 37°C with rocking) with TF (5 pM) alone (closed squares), TF (5 pM) plus PAR1 (100 μM) and PAR4 (500 μM) agonist peptides (closed circles), or TF (5 pM) plus FV (2 nM) (open circles). TAT formation was measured by ELISA as described in “Study design.” For comparison, TAT formation in the presence of PAR agonist peptides was also assessed in 2 unaffected individuals assayed in duplicate (mean ± standard deviation) (open triangles).
The patient’s platelet-derived FV pool remaining 10 days after plasma administration supports thrombin generation in a TF-initiated whole blood clotting model. Whole blood from the patient was incubated (at 37°C with rocking) with TF (5 pM) alone (closed squares), TF (5 pM) plus PAR1 (100 μM) and PAR4 (500 μM) agonist peptides (closed circles), or TF (5 pM) plus FV (2 nM) (open circles). TAT formation was measured by ELISA as described in “Study design.” For comparison, TAT formation in the presence of PAR agonist peptides was also assessed in 2 unaffected individuals assayed in duplicate (mean ± standard deviation) (open triangles).
Following its endocytosis by megakaryocytes, FV is retailored to form a physically distinct molecule that exhibits an increased procoagulant potential.4-10 Because of its localized release from the platelets’ α-granules at vascular injury sites, platelet-derived FV/Va is the predominant cofactor in thrombin generation at the platelet surface.16 Thus, these combined observations suggest that despite a complete absence of a plasma-derived FV and the presence of ∼7% normal levels of platelet-derived FV/Va, the persistence of the highly procoagulant cofactor in the patient’s platelets confers hemostatic competence. The importance of platelets and platelet-derived FV/Va in sustaining normal hemostasis is supported by several studies. A patient with a neutralizing inhibitor to plasma- but not platelet-derived FV showed no bleeding tendency following extensive surgical challenge.16 In contrast, individuals with platelet-derived FV/Va inhibitors exhibit severe GI bleeding.17,18 Other reports describe the success of platelet transfusions in the cessation of severe bleeding resulting from FV deficiency19,20 or FV inhibitors.21-23 In a recent study, Duckers et al described 3 individuals with severe plasma-derived FV/Va deficiency (<1% activity) but expression of detectable platelet-derived FV/Va antigen and activity (1.7% to 6.4%) who exhibited only a mild bleeding diathesis.24 The authors speculate that this residual platelet-derived FV/Va coupled with the decreased TFPI levels observed in these individuals allows for sufficient thrombin generation to prevent fatal bleeding.24 Indeed, our patient’s plasma TFPIα level (5.9 ± 0.65 ng/mL) was dramatically lower than that observed in a normal plasma pool (13.0 ± 0.95 ng/mL) consistent with previous observations made in FV-deficient individuals.24,25
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Robert Hondal who helped with peptide synthesis, Dr Matthew Whelihan for his assistance with the whole blood clotting assays, Dr Jolanta Krudysz-Amblo for her assistance with assay of thrombin-antithrombin III, Fatema Walji for her assay of platelet prothrombinase activity, and Dr Kenneth Mann for his generous donation of anti-human FV #9 and #17.
This work was supported by an American Heart Association Scientist Development Grant (0635048N), the University of Vermont College of Medicine Internal Grant Program (B.A.B.), and the National Institutes of Health National Heart, Lung, and Blood Institute grants HL46703 (Project 3 [P.B.T.] and Project 5 [K.E.B.-Z.]), HL91111 (B.A.B.), T32HL007594 (J.C.), and award UC2 HL103010 (P.D.).
Authorship
Contribution: B.A.B. performed research, analyzed data, and prepared the manuscript; J.C., K.E.B.-Z., and P.D. performed research, analyzed data, and critically reviewed the manuscript; N.S.K. oversaw the participation and clinical management of the patient and critically reviewed the manuscript; and P.B.T. conceived of the research, analyzed data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paula B. Tracy, Department of Biochemistry, University of Vermont, College of Medicine, Given C409, 89 Beaumont Ave, Burlington, VT 05405-0068; e-mail: paula.tracy@uvm.edu.