Key Points
Cytosolic HSP90-bound Lyn mediates resistance to apoptosis by strengthening PP2A/SET interaction in CLL cells.
FTY720-analogues antagonizing the PP2A/SET interaction and Lyn inhibitors may provide a therapeutic approach of CLL.
Abstract
Aberrant protein kinase activities, and the consequent dramatic increase of Ser/Thr and -Tyr phosphorylation, promote the deregulation of the survival pathways in chronic lymphocytic leukemia (CLL), which is crucial to the pathogenesis and progression of the disease. In this study, we show that the tumor suppressor protein phosphatase 2A (PP2A), one of the major Ser/Thr phosphatases, is in an inhibited form because of the synergistic contribution of 2 events, the interaction with its physiologic inhibitor SET and the phosphorylation of Y307 of the catalytic subunit of PP2A. The latter event is mediated by Lyn, a Src family kinase previously found to be overexpressed, delocalized, and constitutively active in CLL cells. This Lyn/PP2A axis accounts for the persistent high level of phosphorylation of the phosphatase’s targets and represents a key connection linking phosphotyrosine- and phosphoserine/threonine-mediated oncogenic signals. The data herein presented show that the disruption of the SET/PP2A complex by a novel FTY720-analog (MP07-66) devoid of immunosuppressive effects leads to the reactivation of PP2A, which in turn triggers apoptosis of CLL cells. When used in combination with SFK inhibitors, the action of MP07-66 is synergistically amplified, providing a new option in the therapeutic strategy for CLL patients.
Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in the Western world, is characterized by the proliferation of CD5+/CD19+/CD23+ B lymphocytes in lymphoid tissues and bone marrow, which progressively accumulate as mature quiescent cells in the peripheral blood.1,2 A key role in proliferation and survival of CLL cells is played by microenvironmental factors, which in turn trigger multiple signals mediated by cell surface receptors including CD40, toll-like receptor and B-cell receptor (BCR).3-5 The efforts toward a therapeutic intervention, besides the standard chemo-immunotherapy, have recently focused on hyperactive kinases downstream of the BCR,6,7 with the development of therapeutically promising inhibitors of these enzymes.8-10
Lyn, for instance, a tyrosine kinase belonging to the Src family kinases (SFKs), has been shown to play a crucial role in the onset and progression of CLL. In CLL cells, Lyn is overexpressed and distributed into 2 pools, one associated beneath the cell surface and the other bound to an aberrant cytosolic complex, which exhibits the constitutive activation responsible for the phosphorylation of a myriad of protein targets in the cytosol and for the resistance of CLL cells to apoptosis.11-13
Because Lyn-targeting drugs, such as dasatinib,14,15 have yielded disappointing results in managing CLL,16-19 we have recently focused on the substrates of the cytosolic pool of Lyn to better dissect the mechanisms contrasting apoptosis and possibly extend the repertoire of pharmacologic targets of this disease.13
In the present study, we used Lyn inhibitors as tools to identify novel players whose function might be altered by the constitutive activity of Lyn and to verify a possible role thereof as therapeutic targets. Importantly, though it is a tyrosine kinase inhibitor, dasatinib is known to cause a drastic decrease in the phosphorylation of specific serine residues of protein kinases that are central to cell survival such as Akt, ERK 1/2, and p38, but not to affect their activity directly.20 In the attempt to explore this phenomenon, we highlighted a novel axis formed by Lyn, the tumor suppressor protein phosphatase 2A (PP2A),21 and the physiologic inhibitor thereof named SET, an oncoprotein overexpressed in CLL,22,23 which contributes to the activation of prosurvival players in CLL cells. Mechanistically, we show that the interaction of PP2A and SET, which per se determines a marked inhibition of the phosphatase activity, is further stabilized by the phosphorylation of PP2A by an aberrant form of Lyn, which ultimately prevents PP2A from switching off the survival signaling network in CLL. Our findings indicate that PP2A is situated at the crossroads of phosphotyrosine and phosphoserine/threonine-mediated oncogenic signals in this disease, and its proapoptotic potential can be fully unleashed by targeting the factors that impair its activity.
Materials and methods
Ethics statement
Written informed consent was obtained from all patients before sample collection and according to the Declaration of Helsinki. The ethical approval for our study was obtained from the local ethical committee of Regione Veneto on Chronic Lymphocytic Leukemia.
Patients, cell separation, and culture conditions
B cells from 42 untreated CLL patients, and 6 normal volunteers were purified and cultured as previously described by our group,24-26 and subjected to the treatments described throughout the text. The patients’ relevant features are reported in supplemental Table 1, available on the Blood Web site.
Coculture conditions
1 × 105/well bone mesenchymal stromal cells from CLL patients were seeded into 12-well plates and incubated for several days before the experiment at 37°C in 5% CO2 up to confluence. Then CLL B cells were added to the MSC layer at a ratio of 20:1, as described elsewhere 27 and subjected to the treatments described throughout the text.
Cell lysis and subcellular fractionation
For total lysates, normal and CLL cells (5 × 105 for each assay) were rapidly lysed as previously described.11,12 For subcellular fractionation, CLL cells (15 × 106 for each assay) were disrupted and homogenized as previously described.12 Homogenates were centrifuged for 10 minutes at 900g (nuclear fraction). The supernatant was then subjected to ultracentrifugation for 1 hour, performed at 105 000g, to separate cytosol from the postnuclear particulate fraction.
Fractionation by centrifugation on glycerol gradient
150 µg of cytosolic proteins from normal B cells and CLL cells were loaded on a glycerol linear gradient (10%-40%), centrifuged, and processed as described elsewhere.12
Synthesis of fingolimod analogs
Fingolimod analogs were prepared by canonical solution synthesis by reacting p-hydroxybenzaldehyde with proper haloalkanes and amines. Standard procedures reported for Williamson reaction28 and reductive amination29 were followed. The compounds were characterized by HRMS (Mariner Applied Biosystems ESI-TOF spectrometer) and NMR (NMR Bruker AVANCE III 400) spectroscopy.
Information concerning reagents, subcellular fractionation, PP2A activity assay, apoptosis assays, western blotting, and statistical analysis is detailed in the supplemental Data.
Results
SFK inhibitors, dasatinib, PP2, and saracatinib cause a decrease in serine/threonine phosphorylation of survival mediators in CLL
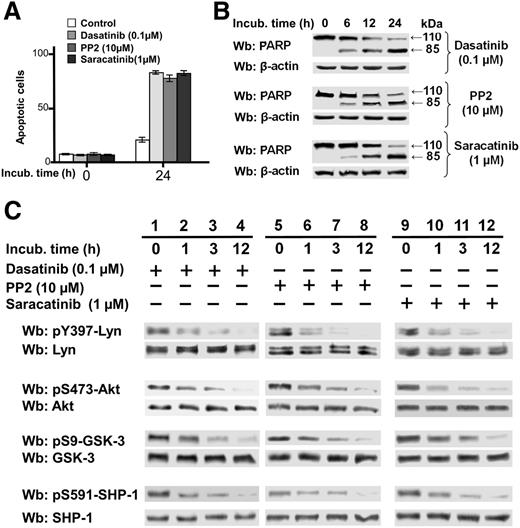
To verify whether SFK inhibitors PP2 and saracatinib30 shared the ability of dasatinib to inhibit Akt—one of the major protein kinases activated—and could affect CLL cell viability, freshly isolated CLL cells from 30 patients were incubated in the absence and presence of dasatinib (0.1 µM), PP2 (10 µM), and saracatinib (1 µM) at different time points. All of these compounds induced cell death, as expected (Figure 1A), by a caspase-dependent mechanism, as confirmed by the cleavage of poly-ADP-ribose polymerase (PARP) (Figure 1B). Phosphorylation of S473 of Akt, which mirrors Akt activation,31 was markedly reduced and so was phosphorylation of S9 of GSK-3, one of the Akt substrates and a major protein kinase regulating the β-catenin stability,32 which instead reflects inhibition of the kinase activity (Figure 1C, middle panels). These findings were concomitant with the decrease of the phosphorylation of Tyr396 in the activation loop of Lyn (Figure 1C, top panel), suggesting that Lyn might affect the regulation of these signaling players in CLL cells. We also focused on SHP-1, a tyrosine phosphatase (PTP) that is segregated into 2 pools in CLL, one being tyrosine-phosphorylated and bound to the plasma membrane in an active form and the other localized to the cytosol in an inhibited conformation as a result of its phosphorylation at S591, and whose action is directed by Lyn to sustain the survival of leukemia cells.33 Under the same experimental conditions, we observed a decrease in the level of phosphorylation of S591, which is indicative of SHP-1 activation and might explain the fall in tyrosine phosphorylation in the cytosol when using SFK inhibitors (Figure 1C, bottom panel).12
Effect of SFK inhibitors on phosphorylation of survival mediators in CLL. (A) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, 10 µM PP2, or 1µM saracatinib for 24 hours, and cell apoptosis was analyzed by annexin V–PI flow cytometry. Quadrant analysis after flow cytometry was expressed as mean percentage ± standard deviation (SD) of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 30 CLL patients. Compared with the control, changes were statistically significant (*P < .01). (B) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, 10 µM PP2, or 1 µM saracatinib at different time points. After such treatment, cells were lysed and analyzed by western blot (Wb) analysis with anti-PARP antibodies, as well as anti-β-actin antibody as a loading control. (C) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, 10 µM PP2, or 1 µM saracatinib at different time points. After such treatment, cells were lysed and analyzed by Wb analysis with antibodies against the phosphorylated form and, after stripping, against the nonphosphorylated form of Lyn, Akt, GSK-3, and SHP-1.
Effect of SFK inhibitors on phosphorylation of survival mediators in CLL. (A) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, 10 µM PP2, or 1µM saracatinib for 24 hours, and cell apoptosis was analyzed by annexin V–PI flow cytometry. Quadrant analysis after flow cytometry was expressed as mean percentage ± standard deviation (SD) of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 30 CLL patients. Compared with the control, changes were statistically significant (*P < .01). (B) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, 10 µM PP2, or 1 µM saracatinib at different time points. After such treatment, cells were lysed and analyzed by western blot (Wb) analysis with anti-PARP antibodies, as well as anti-β-actin antibody as a loading control. (C) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, 10 µM PP2, or 1 µM saracatinib at different time points. After such treatment, cells were lysed and analyzed by Wb analysis with antibodies against the phosphorylated form and, after stripping, against the nonphosphorylated form of Lyn, Akt, GSK-3, and SHP-1.
Serine/threonine phosphatase inhibitors counter the action of SFK inhibitors
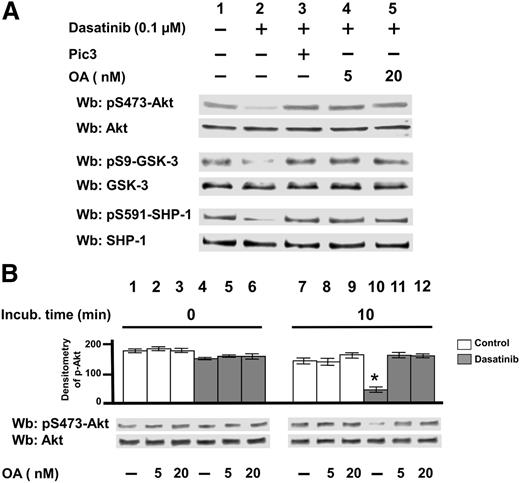
To ascertain a connection between Lyn’s deranged activity and the levels of serine phosphorylation of the aforementioned enzymes in CLL cells, we investigated whether serine/threonine phosphatase activities might be impaired in the CLL background. We therefore incubated freshly isolated CLL cells with dasatinib in the absence or presence of a commercial broad-spectrum phosphatase inhibitor cocktail (Pic3, Sigma-Aldrich) or increasing concentrations of the phosphatase inhibitor okadaic acid (OA), the latter highly specific toward protein phosphatase 2A (PP2A) in the low nanomolar range.34,35 Figure 2A shows that both Pic3 (lane 3) and OA (lanes 4 and 5) could entirely counteract the dephosphorylation of Akt, GSK-3, and SHP-1 induced by dasatinib (lane 2), suggesting that Lyn might contribute to the inhibition of a phosphatase activity. Similar results were achieved by using the SFK inhibitors PP2 and saracatinib (supplemental Figure 1).
Effect of Ser/Thr phosphatase inhibitors on the phosphorylation of survival mediators in CLL. (A) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, supplemented with Pic3 and 5 or 20 nM okadaic acid (OA) for 8 hours. After such treatment, cells were lysed and analyzed by western blot (Wb) analysis with antibodies against the phosphorylated and, after stripping, against the nonphosphorylated forms of Lyn, Akt, GSK-3, and SHP-1. (B) Whole B-cell lysates from 20 CLL patients underwent a phosphatase activity assay in the absence or presence of increasing concentrations of OA by using pAkt immunoprecipitated from CLL cells, for 0 and 10 minutes at 37°C. After such treatment, the samples underwent Wb analysis with anti-pAkt and anti-Akt antibodies. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown. Compared with the control, changes were statistically significant (*P < .01).
Effect of Ser/Thr phosphatase inhibitors on the phosphorylation of survival mediators in CLL. (A) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, supplemented with Pic3 and 5 or 20 nM okadaic acid (OA) for 8 hours. After such treatment, cells were lysed and analyzed by western blot (Wb) analysis with antibodies against the phosphorylated and, after stripping, against the nonphosphorylated forms of Lyn, Akt, GSK-3, and SHP-1. (B) Whole B-cell lysates from 20 CLL patients underwent a phosphatase activity assay in the absence or presence of increasing concentrations of OA by using pAkt immunoprecipitated from CLL cells, for 0 and 10 minutes at 37°C. After such treatment, the samples underwent Wb analysis with anti-pAkt and anti-Akt antibodies. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown. Compared with the control, changes were statistically significant (*P < .01).
To further explore whether Lyn inhibition was able to activate an OA-inhibitable phosphatase, we tested the phosphatase activity, in the presence of increasing concentrations of OA, of total lysates from CLL cells previously untreated or treated with dasatinib (Figure 2B). By using pAkt immunoprecipitated CLL cell lysates as a substrate (Figure 2B), we observed that the phosphatase activity triggered by dasatinib was nearly totally inhibited by OA at a concentration as low as 5 nM, which was confirmed by using a commercial phosphatase assay kit specific for PP2A (supplemental Figure 2). This finding suggested that the protein phosphatase implicated in the dasatinib-induced serine/threonine dephosphorylation might be PP2A. This hypothesis was also consistent with data showing that PP2A is negatively regulated by several tyrosine kinases including members of the Src family,30 which points to PP2A as one of Lyn’s targets in CLL.
PP2A is phosphorylated at Tyr 307 in CLL cells but not in normal B cells
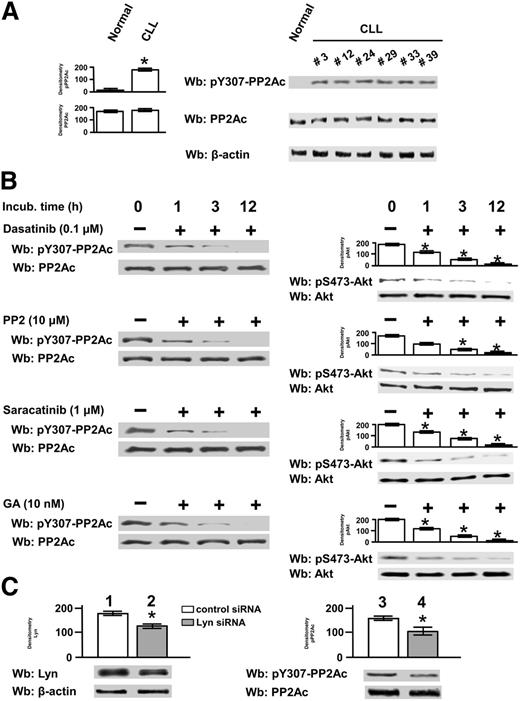
PP2A is a heterotrimer consisting of the 65-kDa structural subunit A, the 36-kDa catalytic subunit C (PP2Ac), and a variable regulatory subunit B. It undergoes sophisticated regulatory mechanisms conferring highly functional specificity, including protein-protein interactions and posttranslational modifications.21,36-39 In particular, endogenous ligands, such as I1PP2A and I2PP2A (the latter also termed SET), can directly bind to PP2Ac and inhibit the phosphatase activity, which has been already observed in CLL.23 In addition, methylation at Leu309 of PP2Ac triggers the phosphatase activity, whereas transient inactivation occurs upon phosphorylation at Y307 of the same subunit.36,37 The latter may be the case in CLL, because, as speculated before, Lyn might inhibit PP2A activity through phosphorylation of Y307 of the catalytic subunit. To evaluate this possibility, CLL cell total lysates of all 42 patients recruited in this study were compared with 6 healthy donors by western blot analysis with anti-phospho-Y307 (pY307)-PP2Ac antibody. As shown in Figure 3A, PP2Ac was constantly phosphorylated in leukemic samples relative to that from healthy donors, which did not undergo phosphorylation even under stimulation with anti-IgM antibody (supplemental Figure 3).
Phosphorylation state and protein level of the catalytic subunit of PP2A in CLL cells. (A) Whole B-cell lysates from 6 normal donors and from all CLL patients were analyzed by western blot (Wb) analysis with anti-pY307-PP2Ac antibody, and the blots were reprobed with anti-PP2Ac antibody as well as anti-β-actin antibody as a loading control (left panel). Wb analysis representative of 1 normal donor and CLL patients (patients 3, 12, 24, 29, 33, and 39) are shown in the right panel. (B) Freshly isolated CLL cells from 14 patients were cultured in the absence or presence of 0.1 µM dasatinib, 10 µM PP2, 1 µM saracatinib, or 10 nM GA at different time points. After such treatment, cells were either (1) lysed and analyzed by Wb analysis with anti-pY307-PP2Ac antibody and the blots were reprobed with anti-PP2Ac antibody (left panels) or (2) subject to a phosphatase activity assay by using pAkt, obtained by immunoprecipitation from CLL cells, for 10 minutes at 37°C. In the latter case, the samples were analyzed by Wb analysis with anti-p-Akt and anti-Akt antibodies. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown (right panels). Compared with the control, changes were statistically significant (*P < .01). (C) Freshly isolated CLL cells were transfected by nucleofection with either the negative control or Lyn-siRNAs and cultured for 48 hours in complete medium. Cell lysates underwent Wb analysis with anti-Lyn antibody (lanes 1and 2) and anti-pY307-PP2A (lanes 3 and 4) and were reprobed, after stripping, with anti-β-actin and anti-PP2Ac antibodies, respectively, as loading controls. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown (right panels). Compared with the control, changes were statistically significant (*P < .01) to assess the efficacy of siRNA technology.
Phosphorylation state and protein level of the catalytic subunit of PP2A in CLL cells. (A) Whole B-cell lysates from 6 normal donors and from all CLL patients were analyzed by western blot (Wb) analysis with anti-pY307-PP2Ac antibody, and the blots were reprobed with anti-PP2Ac antibody as well as anti-β-actin antibody as a loading control (left panel). Wb analysis representative of 1 normal donor and CLL patients (patients 3, 12, 24, 29, 33, and 39) are shown in the right panel. (B) Freshly isolated CLL cells from 14 patients were cultured in the absence or presence of 0.1 µM dasatinib, 10 µM PP2, 1 µM saracatinib, or 10 nM GA at different time points. After such treatment, cells were either (1) lysed and analyzed by Wb analysis with anti-pY307-PP2Ac antibody and the blots were reprobed with anti-PP2Ac antibody (left panels) or (2) subject to a phosphatase activity assay by using pAkt, obtained by immunoprecipitation from CLL cells, for 10 minutes at 37°C. In the latter case, the samples were analyzed by Wb analysis with anti-p-Akt and anti-Akt antibodies. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown (right panels). Compared with the control, changes were statistically significant (*P < .01). (C) Freshly isolated CLL cells were transfected by nucleofection with either the negative control or Lyn-siRNAs and cultured for 48 hours in complete medium. Cell lysates underwent Wb analysis with anti-Lyn antibody (lanes 1and 2) and anti-pY307-PP2A (lanes 3 and 4) and were reprobed, after stripping, with anti-β-actin and anti-PP2Ac antibodies, respectively, as loading controls. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown (right panels). Compared with the control, changes were statistically significant (*P < .01) to assess the efficacy of siRNA technology.
Moreover, Lyn inhibition in CLL cells by dasatinib, PP2, and saracatinib over time brought about a significant decrease in the level of pY307-PP2Ac (Figure 3B, left panels), which effected the elevation of phosphatase activity, as appraised by using pAkt as a substrate (Figure 3B, right panels). To assess whether the cytosolic fraction of Lyn complexed with HSP90 was responsible for PP2Ac phosphorylation, we treated CLL cells with geldanamycin (GA), an Hsp90 inhibitor that had been shown to disassemble the aberrant protein complex and inactivate Lyn.12 As expected, this agent inhibited Lyn activity, proving to be as effective as dasatinib, PP2, and saracatinib in abolishing PP2Ac phosphorylation. Overlapping results were obtained by using the commercial phosphatase assay kit specific for PP2A (supplemental Figure 4). We also attempted to knock down Lyn expression to further substantiate the role of Lyn in PP2A phosphorylation. This treatment did not determine a dramatic decrease in Lyn protein level (Figure 3C, left panel), which is in agreement with our earlier hypothesis that Lyn may undergo a slow turnover because of its association with HSP90 within the cytosol.12 Nevertheless, the phosphorylation of Y307-PP2Ac dropped by ∼30%, which led us to conclude that Lyn is directly implicated in the phosphorylation, and inhibition, of PP2A (Figure 3C, right panel).
Role of phosphorylation of PP2A at Y307 in the association between PP2A and SET
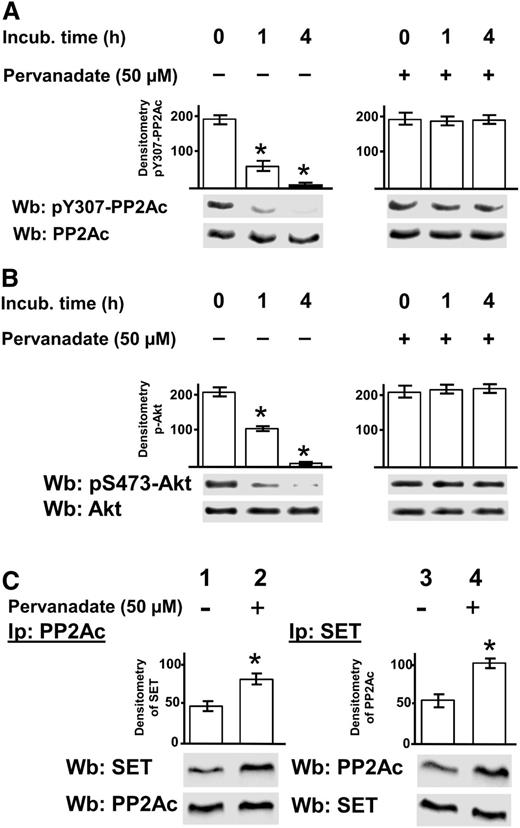
To evaluate the effect of pY307 on the regulation of PP2A, we incubated CLL cells in the absence and presence of pervanadate, an irreversible protein tyrosine phosphatase inhibitor,40 and subsequently estimated the level of phosphorylation of Y307 by western blot analysis performed on whole-cell lysates. Although the treatment with pervanadate prevented dephosphorylation of pY307, the untreated samples exhibited a dramatic decrease in the phosphorylation of this tyrosine residue (Figure 4A).
Role of phosphorylation of PP2Ac at Y307 in the SET/PP2A assembly. Freshly isolated CLL cells were cultured in the absence or presence of 50 µM pervanadate at different time points and subjected to 3 different analyses. (A) The samples were analyzed by western blot (Wb) analysis with anti-pY307-PP2Ac antibody, and the blots were reprobed with anti-PP2Ac antibody. (B) Whole B-cell lysates treated as described before were incubated with pAkt, obtained by immunoprecipitation from CLL cells, for 10 minutes at 37°C, and the samples were subsequently analyzed by Wb analysis with anti-pAkt and anti-Akt antibodies. (C) Each sample underwent immunoprecipitation with anti-PP2Ac antibody (left panel) or anti-SET antibody (right panel), and the immunocomplexes were analyzed by Wb analysis with anti-SET and anti-PP2Ac, respectively. Blots were then stripped and reprobed with the antibody applied for immunoprecipitations. The bar graph above the blot panels represents the values of a densitometric analysis (arbitrary units) of the anti-PP2Ac and anti-SET bands, expressed as ± SD. The pervanadate-treated samples compared with the untreated ones yielded statistically significant changes (*P < .01). Data are representative of experiments performed in triplicate on samples from 20 CLL patients.
Role of phosphorylation of PP2Ac at Y307 in the SET/PP2A assembly. Freshly isolated CLL cells were cultured in the absence or presence of 50 µM pervanadate at different time points and subjected to 3 different analyses. (A) The samples were analyzed by western blot (Wb) analysis with anti-pY307-PP2Ac antibody, and the blots were reprobed with anti-PP2Ac antibody. (B) Whole B-cell lysates treated as described before were incubated with pAkt, obtained by immunoprecipitation from CLL cells, for 10 minutes at 37°C, and the samples were subsequently analyzed by Wb analysis with anti-pAkt and anti-Akt antibodies. (C) Each sample underwent immunoprecipitation with anti-PP2Ac antibody (left panel) or anti-SET antibody (right panel), and the immunocomplexes were analyzed by Wb analysis with anti-SET and anti-PP2Ac, respectively. Blots were then stripped and reprobed with the antibody applied for immunoprecipitations. The bar graph above the blot panels represents the values of a densitometric analysis (arbitrary units) of the anti-PP2Ac and anti-SET bands, expressed as ± SD. The pervanadate-treated samples compared with the untreated ones yielded statistically significant changes (*P < .01). Data are representative of experiments performed in triplicate on samples from 20 CLL patients.
Phosphorylation at Y307 of PP2Ac by Lyn raised the question of whether this posttranslational modification could be inhibitory as such,36 or accompanied by other regulatory mechanisms.37-39 In particular, it has been demonstrated that the PP2A inhibitor SET is overexpressed in primary CLL cells, exerting its oncogenic potential through PP2A inhibition.23 As shown in Figure 4B, there was a close correspondence between the phosphorylation status of Y307 and the phosphatase activity using pAkt as a substrate. In fact, the blockade of protein tyrosine phosphatases in the cell lysates by pervanadate hindered the dephosphorylation of PP2A pY307 and consequently the catalytic activity of PP2A. Moreover, to establish whether the phosphorylative event could be related to the PP2A interaction with SET, we immunoprecipitated PP2Ac, or alternatively SET, from CLL total cell lysates untreated or treated with pervanadate and performed western blot analysis with anti-PP2Ac and anti-SET antibodies. SET and PP2Ac coimmunoprecipitated in a larger amount from the lysates of the pervanadate-treated cells than from the untreated ones (Figure 4C), indicating that the persistence of phosphorylation at Y307 stabilized the protein complex, leading us to speculate that pY307 enhances the inhibitory function of SET on PP2A.
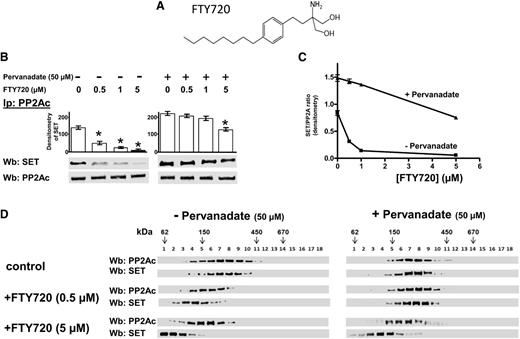
Effect of FTY720 on the association of the PP2A/SET complex
FTY720 (fingolimod, Figure 5A) is a sphingosine analog used as an immunosuppressive drug in the treatment of relapsing-remitting multiple sclerosis and for preventing kidney graft rejection, acting, upon phosphorylation by sphingosine kinase 2, as a functional antagonist of sphingosine-1-phosphate (S1P) receptors (S1PRs).41-43 It also possesses antitumor activity, especially against hematologic malignancies, bringing about apoptosis through PP2A activation by interfering with the interaction of the phosphatase with SET.44 The latter property of FTY720 prompted us to better define the degree to which pY307 contributes to the association between SET and PP2A. Therefore, the cytosol from CLL cells untreated or treated with pervanadate was incubated with increasing concentrations of FTY720 (0-5 µM), and subjected to either immunoprecipitation with anti-PP2A antibody or separation by ultracentrifugation on a linear glycerol gradient, with subsequent western blot analysis with anti-SET and anti-PP2Ac antibodies. FTY720 displaced SET from the PP2A of immunoprecipitates from pervanadate-untreated B-cell CLL cells more effectively than from pervanadate-treated ones (Figure 5B-C). Furthermore, the fractionation of the cytosol of pervanadate-treated and -untreated CLL cells on a glycerol gradient (Figure 5D) revealed that PP2A and SET were detected at a molecular weight peak corresponding to ∼220 kDa in the absence of FTY720, whereas the presence of FTY720 resulted in a different distribution of PP2Ac and SET based on the pretreatment with pervanadate. In fact, in the pervanadate-untreated samples, there was a shift of PP2A and SET to the expected molecular weight of the PP2A holoenzyme and SET as such at the highest concentration of FTY720 (5 µM, left panel), whereas the pervanadate-treated samples exhibited a distribution over molecular weights suggestive of a more efficient assembly of the protein complex (right panel), confirming that Y307 phosphorylation plays a crucial role in stabilizing the PP2A/SET complex.
Effect of FTY720 on the association of the SET/PP2A complex. (A) Chemical structure of FTY720. (B) Freshly isolated CLL cells were cultured in the absence or presence of 50 µM pervanadate, supplemented with increasing concentration of FTY720 for 8 hours. After such treatment, each sample was immunoprecipitated with anti-PP2Ac antibody and the immunocomplexes were analyzed by western blot (Wb) analysis with anti-SET. Blots were then stripped and reprobed with anti-PP2Ac antibody. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown. (C) The interaction of SET and PP2A in pervanadate-treated and untreated CLL cells was measured as a ratio of the densitometric values of SET over those of PP2A as a function of FTY720 concentration. (D) Cytosol purified by freshly isolated CLL cells treated as previously described was loaded on top of a linear glycerol gradient (10%-40%) and centrifuged for 18 hours at 100 000g in an SW60Ti rotor (Beckman Coulter) at 4°C. Eighteen fractions (200 μL each) were collected from top and analyzed by immunoblotting with anti-PP2Ac and anti-SET antibody. The figure is representative of experiments performed in triplicate on samples from each of 16 CLL patients. Downward arrows represent the position of molecular weight standards on glycerol gradients, glutamate dehydrogenase (62 kDa), alcohol dehydrogenase (150 kDa), apoferritin (443 kDa), and thyroglobulin (669 kDa; Sigma-Aldrich), indicated to estimate the molecular weight of the protein. The figure is representative of experiments performed in triplicate on samples from each of 16 CLL patients.
Effect of FTY720 on the association of the SET/PP2A complex. (A) Chemical structure of FTY720. (B) Freshly isolated CLL cells were cultured in the absence or presence of 50 µM pervanadate, supplemented with increasing concentration of FTY720 for 8 hours. After such treatment, each sample was immunoprecipitated with anti-PP2Ac antibody and the immunocomplexes were analyzed by western blot (Wb) analysis with anti-SET. Blots were then stripped and reprobed with anti-PP2Ac antibody. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown. (C) The interaction of SET and PP2A in pervanadate-treated and untreated CLL cells was measured as a ratio of the densitometric values of SET over those of PP2A as a function of FTY720 concentration. (D) Cytosol purified by freshly isolated CLL cells treated as previously described was loaded on top of a linear glycerol gradient (10%-40%) and centrifuged for 18 hours at 100 000g in an SW60Ti rotor (Beckman Coulter) at 4°C. Eighteen fractions (200 μL each) were collected from top and analyzed by immunoblotting with anti-PP2Ac and anti-SET antibody. The figure is representative of experiments performed in triplicate on samples from each of 16 CLL patients. Downward arrows represent the position of molecular weight standards on glycerol gradients, glutamate dehydrogenase (62 kDa), alcohol dehydrogenase (150 kDa), apoferritin (443 kDa), and thyroglobulin (669 kDa; Sigma-Aldrich), indicated to estimate the molecular weight of the protein. The figure is representative of experiments performed in triplicate on samples from each of 16 CLL patients.
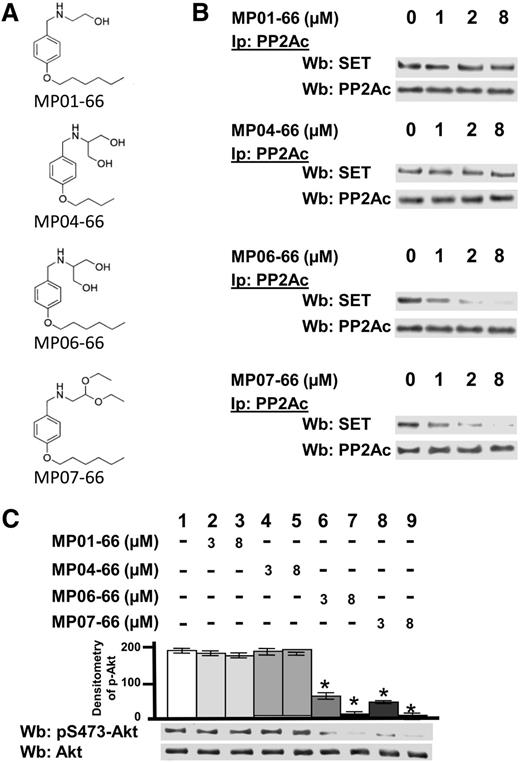
Effect of FTY720 analogs on the stability of the SET/PP2A complex
The immunosuppressive action of FTY720 related to S1PR degradation and subsequent T-cell homing in the secondary lymphoid organs makes this drug unsuitable to be used as an antitumor drug. To circumvent this issue, other authors have developed FTY720 analogs with potent cytotoxicity against specific neoplasms, devoid of sphingosine-1-phosphate receptor-mediated immunosuppressive effects and shown to activate PP2A, thereby unleashing the apoptotic cascades.45,46 Considering these promising findings and the findings herein reported on PP2A in CLL, we designed a number of FTY720 analogs to be tested for their ability to exert antitumor activity by activating PP2A, but insusceptible to sphingosine kinase 2 (SK-2) phosphorylation and hence unable to cause S1P1 internalization.47,48 Figure 6A shows 4 of the 10 FTY720 analogs, which were designed taking into account the structural properties of FTY720 that mediate its effects and were properly modified on the basis of the functional results achieved. MP06-66, the compound most similar to FTY720, and MP07-66 were as effective as FTY720 in displacing SET from PP2A, whereas MP01-66 and MP04-66 left the SET/PP2A complex unaffected (Figure 6B). Considering their capacity to disrupt the SET/PP2A complex, MP06-66 and MP07-66 were also tested for their ability to activate PP2A. These compounds triggered PP2A activity, as demonstrated by the dephosphorylation of pAkt used as a substrate after immunoprecipitation from CLL cell lysates (Figure 6C) or by the commercial phosphatase assay kit (supplemental Figure 5), whereas MP01-66 and MP04-66 did not. We then wanted to assess whether the FTY720-analogs activating PP2A could alter the protein level of S1PRs at the plasma membrane. Of the 4 compounds studied, only MP06-66 behaved similarly to FTY720, causing a decrease of plasma membrane S1P1, which instead was not affected by MP07-66 (supplemental Figure 6). Because our aim was to identify an FTY720 analog capable of only activating PP2A and devoid of immunosuppressive activity, we chose MP07-66 for in-depth analysis of its potential as a proapoptotic compound.
Effect of FTY720 analogs on the stability of the SET/PP2A complex. (A) Chemical structure of FTY720 analogs. (B) Freshly isolated CLL cells were cultured in the absence or presence of increasing concentrations of MP01-66, MP04-66, MP06-66, or MP07-66 for 8 hours. After such treatment, each sample was immunoprecipitated with anti-PP2Ac antibody, and the immunocomplexes were analyzed by western blot (Wb) analysis with anti-SET. Blots were then stripped and reprobed with anti-PP2Ac antibody. (C) Whole B-cell lysates were incubated with pAkt, obtained by immunoprecipitation from CLL cells, in the absence or presence of increasing concentrations of MP01-66, MP04-66, MP06-66, or MP07-66 for 10 minutes at 37°C. After such treatment, the samples were analyzed by Wb analysis with anti-pAkt and anti-Akt antibodies. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown. The figure is representative of experiments performed in triplicate on samples from each of 16 CLL patients.
Effect of FTY720 analogs on the stability of the SET/PP2A complex. (A) Chemical structure of FTY720 analogs. (B) Freshly isolated CLL cells were cultured in the absence or presence of increasing concentrations of MP01-66, MP04-66, MP06-66, or MP07-66 for 8 hours. After such treatment, each sample was immunoprecipitated with anti-PP2Ac antibody, and the immunocomplexes were analyzed by western blot (Wb) analysis with anti-SET. Blots were then stripped and reprobed with anti-PP2Ac antibody. (C) Whole B-cell lysates were incubated with pAkt, obtained by immunoprecipitation from CLL cells, in the absence or presence of increasing concentrations of MP01-66, MP04-66, MP06-66, or MP07-66 for 10 minutes at 37°C. After such treatment, the samples were analyzed by Wb analysis with anti-pAkt and anti-Akt antibodies. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown. The figure is representative of experiments performed in triplicate on samples from each of 16 CLL patients.
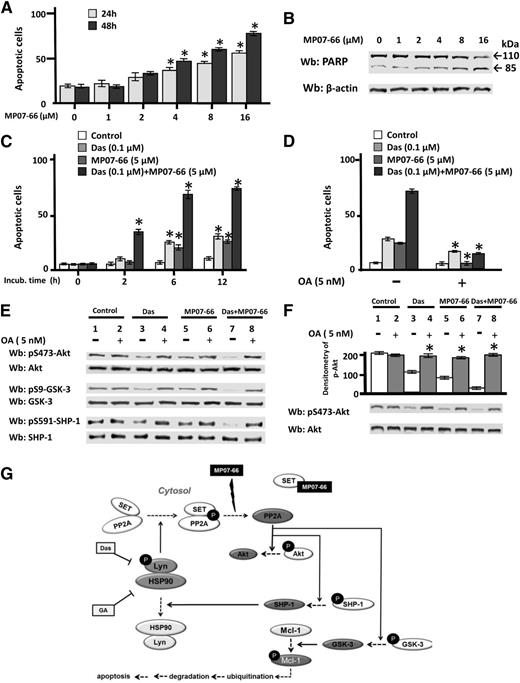
Dasatinib potentiates the proapoptotic effect of MP07-66 in a synergistic manner
Because MP07-66, though not bringing about internalization of S1PRs, acted similar to FTY720 and MP06-66 toward the SET/PP2A complex, we tested MP07-66 for another critical property related to FTY720, which is the ability to induce apoptosis in CLL cells.49 CLL cells were incubated with increasing concentrations of MP07-66 (0-16 µM) for 24 and 48 hours and then subject to annexin V–PI flow cytometry. As shown in Figure 7A, the level of apoptosis reached 50% and 80%, at 24 hours and 48 hours, respectively, at a MP07-66 concentration as high as 16 µM, which paralleled PARP cleavage (Figure 7B), a marker of caspase-dependent apoptosis. In addition, the phosphorylation status of PP2A targets, namely Akt, GSK-3, and SHP-1, was negatively affected by the treatment with MP07-66, triggering known downstream events promoting apoptosis, such as Mcl-1 degradation and caspase-3 activation50,51 (supplemental Figure 7). Notably, it is not surprising that under these experimental conditions Lyn turned out to be deactivated, considering that earlier evidence highlighted the pivotal role of PP2A in suppressing Lyn activity via SHP-1 activation,52 which is further corroborated by the recovery of Lyn autophosphorylation by contrasting the effect of MP07-66 with OA and a SHP-1 inhibitor (PTP-I-I) (supplemental Figure 8).
Effect of the combined action (synergism) of dasatinib and MP07-66 on CLL cell survival. (A) Freshly isolated CLL cells from all the 42 patients recruited in this study (see supplemental Table 1 for details) were cultured in the absence or presence of increasing concentrations of MP07-66 at different time points, and cell apoptosis was analyzed by annexin V–PI flow cytometry. Quadrant analysis after flow cytometry was histogrammed and expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 20 CLL patients. Compared with the control, changes were statistically significant (* P < .01). (B) Freshly isolated CLL cells were cultured in the absence or presence of increased concentration of MP07-66 at different time points. After such treatment, cells were lysed and analyzed by western blot (Wb) analysis with anti-PARP antibody, as well as anti-β-actin antibody as a loading control. (C) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, 5 µM MP07-66, or a combination of 0.1 µM dasatinib and 5 µM MP07-66 at different time points, and cell apoptosis was analyzed by annexin V–PI flow cytometry. Quadrant analysis after flow cytometry was histogrammed and expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 20 CLL patients. Compared with the effect of the compounds used alone, changes were statistically significant (*P < .01). (D) Freshly isolated CLL cells were cultured as described in (C) in the absence or presence of 5 nM of OA for 6 hours, and cell apoptosis was analyzed by annexin V–PI flow cytometry. Quadrant analysis after flow cytometry was histogrammed and expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 20 CLL patients. Compared with the effect of the compounds used alone, changes were statistically significant (* P < .01). (E) Freshly isolated CLL cells were cultured in the absence or presence of 5 nM OA and treated with 1 µM dasatinib, 5 µM MP07-66, or both, for 6 hours. After such treatment, cells were lysed and analyzed by Wb analysis with antibodies against the phosphorylated form and, after stripping, the nonphosphorylated form of Akt, GSK-3, and SHP-1. (F) Freshly isolated CLL cells were cultured in the presence of 5 nM OA and treated with 1 µM dasatinib, 5 µM MP07-66, or both, for 6 hours. After such treatment, cells were lysed and underwent a phosphatase activity assay by using pAkt immunoprecipitated from CLL cells, in the absence or presence of 5 nM OA for 10 minutes at 37°C. After such treatment, the samples underwent Wb analysis with anti-pAkt and anti-Akt antibodies. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown. Compared with the control, changes were statistically significant (*P < .01). (G) A schematic representation of the altered signaling network sustained by the Lyn-PP2A axis in CLL. Lyn, by phosphorylating PP2A, stabilizes the SET/PP2A complex and prevents dephosphorylation of downstream players (Akt, GSK-3, and SHP-1) and ultimately the onset of apoptosis. This latter event is initiated by small molecules affecting either Lyn activity (dasatinib), the stability of the aberrant cytosolic Lyn/Hsp90 complex (geldanamycin), or of the SET/PP2A complex (MP07-66), eventually resulting in the degradation of antiapoptotic proteins, which in turn triggers caspase-dependent apoptosis. Solid lines with arrows denote action; dotted lines with arrows denote process; solid lines with bars denote inhibition.
Effect of the combined action (synergism) of dasatinib and MP07-66 on CLL cell survival. (A) Freshly isolated CLL cells from all the 42 patients recruited in this study (see supplemental Table 1 for details) were cultured in the absence or presence of increasing concentrations of MP07-66 at different time points, and cell apoptosis was analyzed by annexin V–PI flow cytometry. Quadrant analysis after flow cytometry was histogrammed and expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 20 CLL patients. Compared with the control, changes were statistically significant (* P < .01). (B) Freshly isolated CLL cells were cultured in the absence or presence of increased concentration of MP07-66 at different time points. After such treatment, cells were lysed and analyzed by western blot (Wb) analysis with anti-PARP antibody, as well as anti-β-actin antibody as a loading control. (C) Freshly isolated CLL cells were cultured in the absence or presence of 0.1 µM dasatinib, 5 µM MP07-66, or a combination of 0.1 µM dasatinib and 5 µM MP07-66 at different time points, and cell apoptosis was analyzed by annexin V–PI flow cytometry. Quadrant analysis after flow cytometry was histogrammed and expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 20 CLL patients. Compared with the effect of the compounds used alone, changes were statistically significant (*P < .01). (D) Freshly isolated CLL cells were cultured as described in (C) in the absence or presence of 5 nM of OA for 6 hours, and cell apoptosis was analyzed by annexin V–PI flow cytometry. Quadrant analysis after flow cytometry was histogrammed and expressed as mean percentage ± SD of early and late apoptosis from 3 separate experiments performed in triplicate on samples from 20 CLL patients. Compared with the effect of the compounds used alone, changes were statistically significant (* P < .01). (E) Freshly isolated CLL cells were cultured in the absence or presence of 5 nM OA and treated with 1 µM dasatinib, 5 µM MP07-66, or both, for 6 hours. After such treatment, cells were lysed and analyzed by Wb analysis with antibodies against the phosphorylated form and, after stripping, the nonphosphorylated form of Akt, GSK-3, and SHP-1. (F) Freshly isolated CLL cells were cultured in the presence of 5 nM OA and treated with 1 µM dasatinib, 5 µM MP07-66, or both, for 6 hours. After such treatment, cells were lysed and underwent a phosphatase activity assay by using pAkt immunoprecipitated from CLL cells, in the absence or presence of 5 nM OA for 10 minutes at 37°C. After such treatment, the samples underwent Wb analysis with anti-pAkt and anti-Akt antibodies. Pooled densitometric analyses (arbitrary units) of the Wb bands of the immunoblots are shown. Compared with the control, changes were statistically significant (*P < .01). (G) A schematic representation of the altered signaling network sustained by the Lyn-PP2A axis in CLL. Lyn, by phosphorylating PP2A, stabilizes the SET/PP2A complex and prevents dephosphorylation of downstream players (Akt, GSK-3, and SHP-1) and ultimately the onset of apoptosis. This latter event is initiated by small molecules affecting either Lyn activity (dasatinib), the stability of the aberrant cytosolic Lyn/Hsp90 complex (geldanamycin), or of the SET/PP2A complex (MP07-66), eventually resulting in the degradation of antiapoptotic proteins, which in turn triggers caspase-dependent apoptosis. Solid lines with arrows denote action; dotted lines with arrows denote process; solid lines with bars denote inhibition.
These results along with the demonstration that MP07-66 disrupted the SET/PP2A complex, with consequent activation of PP2A (Figure 6), and that Lyn-dependent phosphorylation contributes to the stabilization of the SET/PP2A complex itself (Figure 5), prompted us to use MP07-66 in combination with dasatinib, Lyn inhibitor, on CLL cells and evaluate the death rate over time. Dasatinib (0.1 µM) and MP07-66 (5 µM) proved scarcely efficient when used alone unless CLL cells were incubated for at least 24 hours, whereas combining the two drugs resulted in a death rate as a high as 70% at 6 hours (Figure 7B, left panel). Similar results, both with the compounds used alone or in combination, were obtained by using the coculture of CLL cells with bone marrow mesenchymal stromal cells, a model much closer to CLL pathophysiology (supplemental Figure 9). This 6-hour time point was further analyzed to confirm that PP2A activation was involved in the apoptotic process by using OA. As expected, 5 nM OA drastically reduced the apoptotic rate of CLL cells treated with dasatinib, MP07-66 or the combination of both (Figure 7D), corroborating the hypothesis that inhibition of PP2A is central to CLL cell viability. The role of PP2A was further explored by lysing CLL cells treated as described before and subsequently by evaluating the phosphorylation status of its substrates Akt, GSK-3, and SHP-1, as well as the phosphatase activity. Again, the combined treatment of dasatinib and MP07-66 was the most efficient in provoking a decrease in the phosphorylation of PP2A substrates compared with the single agents (Figure 7E) and inhibiting the phosphatase activity, tested on pAkt immunoprecipitated from CLL cells (Figure 7F), or by the commercial phosphatase assay kit (supplemental Figure 10), which effects were nearly completely counteracted by OA. These results indicate that inhibition of PP2A can be regarded as a novel antiapoptotic mechanism, to which Lyn’s aberrant activity significantly contributes, in CLL cells.
Discussion
In the present study, we show that in CLL the phosphorylation of Y307 of the catalytic subunit of PP2A (PP2Ac) by the aberrant cytosolic form of Lyn stabilizes the interaction of PP2A with its physiologic inhibitor SET. This event significantly contributes to the suppression of the activity of PP2A itself and consequently to the persistent serine/threonine phosphorylation of PP2A substrates that mediate prosurvival and antiapoptotic signals.
Known as a tumor suppressor regulating a wide variety of cellular processes,21,36-39 PP2A undergoes a complex regulation that strictly depends on a fine balance in the composition of its heterotrimeric structure of PP2A, posttranslational modifications affecting each single subunit, and the interaction with protein binding partners. Alterations of one or more such mechanisms may lead to inactivation of PP2A, which commonly occurs in many solid and hematopoietic malignancies including CLL.23,44,48,53,54 Overexpression of SET, in particular, is a recurrent factor associated with PP2A impairment and a poor prognostic factor in such diseases.55 In this scenario, molecules interfering with SET/PP2A association, including the sphingosine analog FTY720 or analogs thereof39,40 and SET peptide antagonists41 have already yielded promising results in vitro and animal models of various types of neoplasms.45,53,54
Our data confirm the role of the aforementioned assembly in PP2A inactivation and extend this observation, demonstrating that in CLL cells, an additional mechanism hampering the phosphatase activity is represented by the phosphorylation of PP2Ac at Y307 by the SFK Lyn. This latter was previously found to be overexpressed and part of an aberrant cytosolic multiprotein complex exhibiting high constitutive activity, accounting for the abnormal level of tyrosine phosphorylation in CLL cells.11 Accordingly, SFK inhibitors such as dasatinib, PP2, and saracatinib bring about a decrease in the level of phosphorylation of Y307, with consequent activation of the phosphatase activity, in turn resulting in the dephosphorylation of the PP2A substrates monitored in this work (Akt, GSK-3, and SHP-1). Importantly, the PP2A-dependent activation of SHP-1 results in the deactivation Lyn, thereby breaking the vicious circle that is governed by the aberrant activity of Lyn itself, ultimately tuning down prosurvival pathways. Notably, overlapping effects were observed using geldanamycin, an HSP90 inhibitor, which induces dissociation of the anomalous Lyn cytosolic complex,12 indicating that the phosphorylation, and as a result the inhibition, of PP2A mainly depends on the hyperactive cytosolic pool of Lyn. Interestingly, pY307 not only appears to have a role in impairing PP2A activity, but also, by a yet-to-be-defined mechanism, facilitates the formation and strengthening of the stability of the SET/PP2A complex. In fact, when using FTY720 as a tool for testing the stability of the SET/PP2A complex, the complex turned out to be more stable when tyrosine phosphorylation was preserved, compared with the experimental conditions where this option was not applied.
In the attempt to translate these findings into new approaches in the treatment of CLL, novel nonimmunosuppressive FTY720 analogs were synthesized and tested for their ability to antagonize SET and activate PP2A. Although FTY720 as such is capable of disassembling the SET/PP2A complex, its use as antineoplastic drug would be limited by the immunosuppressive action as a result of its metabolic transformation to a phosphorylated derivative, which causes internalization of S1P receptors and subsequently lymphocyte homing.47 Of the 10 analogs examined, MP07-66 lacks the structural determinants for being phosphorylated by SK-2, but is still able to target SET and reactivate PP2A. Importantly, that apoptosis of CLL cells is induced by MP07-66 via PP2A activation is confirmed by the ability of OA to counteract cell death (Figure 7). These data are in line with recent evidence showing that nonphosphorylatable FTY720 analogs unleash apoptosis in a number of cancers by such a mechanism.44,46,51,56 Moreover, the use of MP07-66 in combination with dasatinib results in the amplification of CLL apoptosis in a synergistic manner, supporting our hypothesis that 2 different inhibitory events (ie, tyrosine phosphorylation and interaction with SET) converge onto a single target, PP2A, and closely cooperate in generating a vicious circle that maintains the survival of CLL. Of note, this is in agreement with other studies regarding other types of leukemia, including acute myeloid leukemia and chronic myeloid leukemia, where the synergistic action of tyrosine kinase inhibitors and SET antagonists in inducing apoptosis has been observed, and a combination of these agents has been proposed as a therapeutic approach for patients with disease persistence or tyrosine kinase inhibitor resistance.56
Overall, the evidence provided here adds a piece of knowledge of the complex regulation of PP2A and of how this latter is inserted within an intimate crosstalk, whereby the aberrant cytosolic activity of Lyn is inherently involved, between phosphotyrosine and phosphoserine/threonine-mediated signals that preserves the leukemic status (Figure 7G).
In conclusion, this study proposes PP2A as a potential novel therapeutic target in CLL therapy and confirms the key role of Lyn in supporting the oncogenic signals of this disease. In this scenario, the FTY720 analogs devoid of immunosuppressive effects, in combination with SFK inhibitors, may pave the way to new strategies for the treatment of CLL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by A.I.R.C. (Milan) (G.S., L.T.), MIUR (L.T.), Università degli Studi di Padova (L.T.) (progetto di ateneo), Regione Veneto on Chronic Lymphocytic Leukemia, AIRC Regional Project with Fondazione CARIPARO and CARIVERONA, and FIRB (Rome).
Authorship
Contribution: F.Z. performed the majority of the in vitro research; M.A.P. performed some of the in vitro research and wrote and reviewed the manuscript; L.T. provided clinical patient samples, analyzed the data, and wrote parts of the manuscript; E.T. and L.B. performed some of the in vitro research; F.F., V.T., and M.F. provided clinical patient samples and performed some of the in vitro research; G.Z. designed the FTY720-analogues used in this study; G.R. and V.P. synthesized and purified the FTY720-analogues used in this study; G.S. provided intellectual input into the lymphocyte studies and reviewed the manuscript; and A.M.B. designed the research, reviewed and analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anna Maria Brunati, Department of Molecular Medicine, University of Padua, Viale G. Colombo 3, 35131 Padua, Italy; e-mail: annamaria.brunati@unipd.it; Livio Trentin, Department of Medicine, University of Padua, Via Giustiniani 2, 35128 Padua, Italy; e-mail: livio.trentin@unipd.it.
References
Author notes
F.Z. and M.A.P. contributed equally to this study.