To the editor:
We read with interest the articles published by Calvez et al and Collins et al1,2 reporting an increased inhibitor rate in previously untreated patients (PUPs) with severe hemophilia A receiving Kogenate FS compared with those receiving Advate.
These results somehow extended findings reported in the Research of Determinants of Inhibitor Development (RODIN) study,3 and more recently, another study has been published on this topic.4 Given similarities in the study design and population enrolled in the 4 studies, their results can be pooled together to provide an aggregate estimation of the inhibitor rate in this population.
Incidence rate (IR) and pooled odds ratio (OR) with 95% confidence intervals (CI) were calculated using a random-effect model, and heterogeneity was evaluated using I2 statistics.
Separate analyses have been performed for inhibitors and high-titer (HT) inhibitors. To avoid duplicating data, patients enrolled in the RODIN3 study were excluded from the other studies.1,2
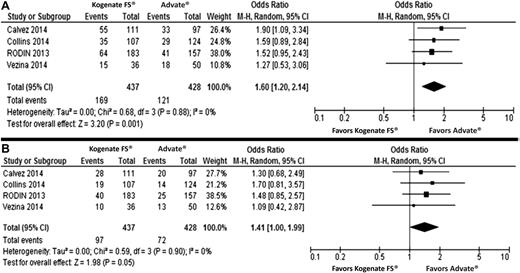
To compare the crude IR between Kogenate FS and Advate, we calculated pooled OR instead of RR to allow an easier comparison with results of the adjusted analysis. A total of 865 PUPs (437 Kogenate FS, 428 Advate) were evaluated, with a follow-up period between 20 and 75 exposure days. Two hundred ninety patients developed inhibitors, with 169 HT inhibitors.
The IR of inhibitors was 0.393 (95% CI: 0.31, 0.48; I2: 63.5%; P = .042) and 0.288 (95% CI: 0.24, 0.34; I2: 37.9%; P = .185) for Kogenate FS and Advate, respectively. For HT inhibitors, the IR was 0.224 (95% CI: 0.19, 0.26; I2: 0%; P = .484) and 0.176 (95% CI: 0.13, 0.24; I2: 54.6%; P = .086) for Kogenate FS and Advate, respectively. The heterogeneity found for IR of inhibitors in Kogenate FS was not confirmed after excluding the study by Calvez et al1 (IR: 0.35; 95% CI: 0.30, 0.40; I2: 0%; P = .623).
A higher rate of inhibitors and a marginally increased rate of HT inhibitors were found in PUPs treated with Kogenate FS (Figure 1). However, none of the studies reported a statistically significant difference among Kogenate FS and Advate in the HT inhibitor rate (Figure 1).
Forest plots of the unadjusted risk of total inhibitor development. (A) High-titer inhibitor development; (B) development in previously untreated patients receiving Kogenate FS or Advate. The analysis on pooled adjusted ORs for inhibitors and high-titer inhibitor development is reported in the text.
Forest plots of the unadjusted risk of total inhibitor development. (A) High-titer inhibitor development; (B) development in previously untreated patients receiving Kogenate FS or Advate. The analysis on pooled adjusted ORs for inhibitors and high-titer inhibitor development is reported in the text.
To account for clinical and demographic characteristics affecting the inhibitor risk, we repeated the analyses by pooling the adjusted ORs obtained by multivariate analyses reported in 3 studies.1-3 A higher rate of inhibitors and HT inhibitors in PUPs treated with Kogenate FS compared with those receiving Advate was found (OR: 1.59; 95% CI: 1.22, 2.08; P = .001, and OR: 1.76; 95% CI: 1.24, 2.50; P = .002, respectively). However, a statistically significant difference among the 2 recombinant factor VIII (rFVIII) groups was reported only in the RODIN study,3 in line with a subgroup analysis of the study by Collins et al,2 suggesting that, among the PUP population in the United Kingdom, those enrolled in the RODIN study showed an increased inhibitor risk compared with the others. Accordingly, in the study by Collins et al,2 the difference in the inhibitor risk between Kogenate FS and Advate was no longer significant when the RODIN population was excluded. These findings and the evidence that in the RODIN study,3 differences were significant in the adjusted but not the unadjusted analysis, might suggest the presence of a selection bias, likely affecting the results.
As a further confirmation, differences between Kogenate FS and Advate were not statistically significant in the study by Calvez et al,1 in which the authors excluded by protocol patients enrolled in the RODIN study.3
Finally, although data from the European Haemophilia Safety Surveillance (EUHASS) project5 were not included in the present analysis to avoid duplicating data, it is noteworthy that no difference in the rate of inhibitor development between Kogenate FS and Advate is reported in this registry (OR: 1.25; 95% CI: 0.74, 2.09; P = .398).
The present meta-analysis, by pooling together data on 865 PUPs, provides information about the IR of inhibitors and HT inhibitors. We evaluated differences in inhibitor rate between Kogenate FS and Advate, and results suggest that some confounding factors may have affected the effect size. The major limitation of our meta-analysis is that, because it was based on aggregate data, we could not address any residual confounding better than the original studies did. An individual patient-level meta-analysis could be performed to better adjust for a series of potential confounders, including variability between studies and centers. However, only a randomized controlled trial could provide a robust answer to the question raised by this body of evidence. Further data are needed before a definite opinion can be given concerning the risk of inhibitor development in patients receiving different types of rFVIII.6
Authorship
Contribution: M.N.D.D.M. conceived and designed the study, performed statistical analysis, interpreted results, and drafted the manuscript; E.M. conceived and designed the study, acquired data, drafted the manuscript, and performed critical revisions; L.V. acquired data and drafted the manuscript; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matteo Nicola Dario Di Minno, Department of Clinical Medicine and Surgery, Federico II University, Via S. Pansini 5, 80131 Naples, Italy; e-mail address: dario.diminno@hotmail.it.