In this issue of Blood, Lorenz et al elucidate the mechanisms by which antibody-mediated targeting of platelet C-type lectinlike receptor 2 (CLEC-2) induces receptor downregulation and thrombocytopenia. This information is important because antibody-mediated targeting of CLEC-2 may have therapeutic utility as antithrombotic therapy, especially if thrombocytopenia can be avoided.1
Excessive platelet aggregation at sites of atherosclerotic plaque rupture can result in formation of pathological thrombi that reduce or obstruct blood flow to downstream tissues and cause tissue ischemia or infarction.2 Consequently, antiplatelet drugs have been developed for treatment of acute thrombotic events. Platelet activation and aggregation at sites of vessel damage is, however, also normally required for cessation of bleeding. It is therefore not surprising that antithrombotic therapies that target platelets are often associated with a disconcertingly high risk for bleeding. The search therefore continues for antithrombotic therapies that do not cause bleeding.
The multiple cell surface receptors that are capable of activating platelets fall into 2 main categories depending on whether they signal through activation of heterotrimeric G proteins, such as Gαq or Gαi, or nonreceptor tyrosine kinases (NRTKs) such as Src-family kinases (SFKs) and spleen tyrosine kinase (Syk).3 The major platelet G-protein–coupled receptors (GPCRs) include the P2Y1 and P2Y12 receptors for adenosine 5′-diphosphate (ADP), the protease-activated receptors (PARs) for thrombin (PAR1 and PAR3 or 4), and the thromboxane/prostaglandin endoperoxide receptor for thromboxane A2. Antiplatelet agents that target the major GPCRs are currently in use, and an elevated risk for bleeding is a well-known side effect associated with each of them.4 The major NRTK-coupled platelet-activating receptors include the glycoprotein VI (GPVI)/Fc receptor γ-chain (GPVI/FcRγ) collagen receptor complex, the C-type lectinlike receptor for podoplanin, CLEC-2, and (in humans) the low-affinity receptor for the Fc portion of the immunoglobulin γ heavy chain, FcγRIIA.5 The first 2 of these receptors represent especially interesting targets for antithrombotic therapy because knockout mice whose platelets fail to express either the GPVI/FcRγ complex or CLEC-2 exhibit impaired thrombus formation in experimental models of arterial injury, but do not bleed more than their wild-type counterparts.6 Efforts to develop and test antibodies or small-molecule inhibitors of GPVI/FcRγ complexes or CLEC-2 for use as antithrombotic agents are therefore currently under way.
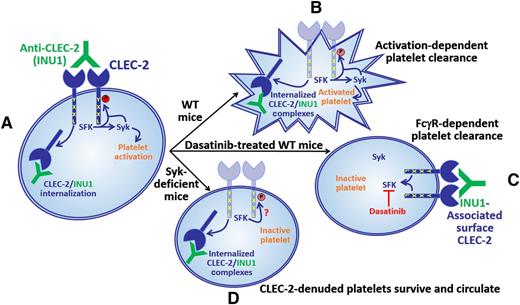
A rat monoclonal antibody, INU1, that is specific for mouse CLEC-2 induces activation of SFK and Syk, resulting in platelet activation and internalization of INU1/CLEC-2 complexes (A). In wild-type mice, INU1-bound platelets, which have internalized their INU1/CLEC-2 complexes, are cleared in a manner that depends upon platelet activation (B). In wild-type mice treated with the SFK inhibitor, dasatinib, platelets can neither be activated nor can they internalize CLEC-2/INU1 complexes; consequently, they are cleared in a manner that depends on binding of FcγRs, presumably on phagocytes, to the Fc region of the INU1 IgG heavy chain (C). Interestingly, in Syk-deficient mice, platelets do not become activated but do internalize CLEC-2/INU1 complexes; consequently, they cannot be cleared by either the activation-dependent or FcγR-dependent pathway and therefore continue to circulate (D). These findings show that antibody-mediated CLEC-2 downregulation can be uncoupled from antibody-induced thrombocytopenia, which is a prerequisite for CLEC-2 targeting strategies to be useful as antithrombotic therapies.
A rat monoclonal antibody, INU1, that is specific for mouse CLEC-2 induces activation of SFK and Syk, resulting in platelet activation and internalization of INU1/CLEC-2 complexes (A). In wild-type mice, INU1-bound platelets, which have internalized their INU1/CLEC-2 complexes, are cleared in a manner that depends upon platelet activation (B). In wild-type mice treated with the SFK inhibitor, dasatinib, platelets can neither be activated nor can they internalize CLEC-2/INU1 complexes; consequently, they are cleared in a manner that depends on binding of FcγRs, presumably on phagocytes, to the Fc region of the INU1 IgG heavy chain (C). Interestingly, in Syk-deficient mice, platelets do not become activated but do internalize CLEC-2/INU1 complexes; consequently, they cannot be cleared by either the activation-dependent or FcγR-dependent pathway and therefore continue to circulate (D). These findings show that antibody-mediated CLEC-2 downregulation can be uncoupled from antibody-induced thrombocytopenia, which is a prerequisite for CLEC-2 targeting strategies to be useful as antithrombotic therapies.
Antibodies that target the GPVI/FcRγ complex or CLEC-2 work to limit thrombosis by inducing loss of the relevant receptor from the surfaces of megakaryocytes and circulating platelets in vivo.6 Receptor downregulation is, however, preceded by a transient, but profound, thrombocytopenia, which causes bleeding and therefore limits the use of these antibodies as antithrombotic agents. Determining the mechanisms underlying antibody-induced receptor downregulation and platelet clearance is important to enable efforts to uncouple the desired effect of receptor downregulation from the undesired effect of thrombocytopenia. The studies by Lorenz et al1 demonstrate that, unlike the GPVI/FcRγ chain complex (which is lost from the surfaces of platelets and megakaryocytes primarily as a consequence of antibody-induced, matrix metalloprotease–dependent ectodomain shedding), antibody-induced downregulation of CLEC-2 is due to internalization of antibody/CLEC-2 complexes, which interestingly requires SFK, but not Syk, activity (see figure). The authors additionally show that the CLEC-2–specific monoclonal antibody, INU1, can induce thrombocytopenia in 2 distinct ways. The first mechanism applies to INU1-treated wild-type mice, in which platelets both become activated and internalize the CLEC-2/INU1 complexes that form on their surfaces (see figure, panel B). Because these platelets internalize CLEC-2/INU1 complexes, their clearance does not involve FcγR-dependent recognition. The precise mechanism by which these activated platelets are cleared remains to be determined. The second mechanism applies to INU1-treated wild-type mice that were also treated with the SFK inhibitor, dasatinib. Platelets in dasatinib-treated mice cannot be activated; however, they also cannot internalize CLEC-2/INU1 complexes and therefore end up being cleared in an FcγR-dependent manner (see figure, panel C). Perhaps the most interesting finding of the study is what happens in INU1-treated Syk-deficient mice, in which platelets do not become activated but do internalize CLEC-2/INU1 complexes (see figure, panel D). These platelets cannot be cleared by either the activation-dependent or the FcγR-dependent pathway and therefore continue to circulate. Thus, Syk deficiency uncoupled the undesired effect of thrombocytopenia from the desired effect of CLEC-2 downregulation in INU1-treated mice. These results suggest that combination therapy with a CLEC-2–specific antibody and a Syk inhibitor, one of which is currently in clinical trials for treatment of rheumatoid arthritis,7 may effectively limit thrombosis without causing significant bleeding.
The finding that INU1-dependent CLEC-2 internalization depends on SFK activity but not Syk was unexpected and has interesting implications for our understanding of events that are most proximal to CLEC-2 engagement. Transduction of activating signals by CLEC-2 requires phosphorylation of a cytoplasmic tyrosine (Y) residue that together with a nearby leucine (L) residue constitutes half (YxxL) of an immunoreceptor tyrosine-based activation motif (hemITAM).5 Signaling by receptors that contain full ITAMs [consensus sequence: YxxL(x6-12)YxxL], such as the GPVI/FcRγ complex, normally involves SFK-mediated phosphorylation of the 2 ITAM tyrosine residues, which then support recruitment of Syk via its tandem Src homology 2 domains and subsequent SFK-dependent Syk phosphorylation and activation.5 CLEC-2 is unique in that its hemITAM has been reported to be phosphorylated primarily by Syk, which requires SFK for activation.8 The finding by Lorenz et al that INU1-induced CLEC-2 internalization requires SFK, but not Syk, activity suggests that either the hemITAM of CLEC-2 can be phosphorylated by SFKs, at least in response to INU1 binding, or that INU1 binding to CLEC-2 induces activation of SFK that contributes in some way other than hemITAM phosphorylation to CLEC-2 internalization (see figure, panel D).
Although CLEC-2 and GPVI are similar to one another in that they both signal through activation of SFKs and Syk, they differ in important ways. Thus, whereas GPVI expression is limited to platelets, CLEC-2 has been reported to be expressed, albeit to a lesser extent, on cells other than platelets, including liver sinusoidal endothelial and Kupffer cells as well as neutrophils and macrophages.9 In addition, whereas GPVI appears to function only in hemostasis and thrombosis, CLEC-2 plays additional roles in other physiological processes that range from lymphangiogenesis and maintenance of blood and lymphatic vessel integrity to organ development (eg, brain, kidney, and lung) and tumor metastasis.9,10 To the extent that efforts to treat thrombotic events by targeting CLEC-2 continue, these additional aspects of CLEC-2 biology must be taken into account.
Conflict-of-interest disclosure: The author declares no competing financial interests.