In this issue of Blood, Peffault de Latour et al describe ex vivo measurements of complement activity in paroxysmal nocturnal hemoglobinuria (PNH) patients on eculizumab treatment. This is the first systematic pharmacodynamic (PD) study of eculizumab in PNH patients which shows that CH50 is a promising biomarker of therapeutic complement blockade.1
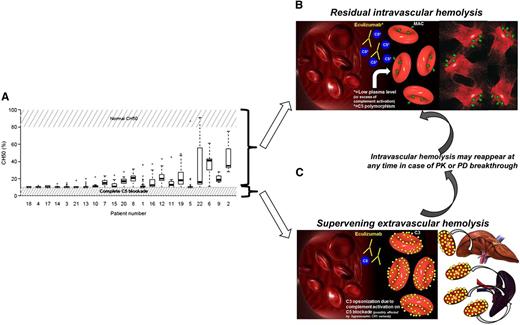
Detection of residual complement activity in PNH patients on eculizumab, with their pathogenic and therapeutic implications. (A) Residual CH50 activity in PNH on eculizumab (see Figure 2A in the article by Peffault de Latour et al that begins on page 775). (B) Mechanism of residual intravascular hemolysis on eculizumab: lack of complete C5 blockade may be due to low plasma level of eculizumab (PK breakthrough) and extra complement activation (PD breakthrough) or rare C5 polymorphisms. Residual intravascular hemolysis may benefit from changes in eculizumab schedule and novel terminal complement inhibitors.10 (C) Mechanism of supervening extravascular hemolysis: due to the lack of CD55, proximal complement activation on RBC remains impaired, eventually leading to C3 opsonization and subsequent C3-mediated extravascular hemolysis.6 C3-mediated extravascular hemolysis may benefit from novel inhibitors of the proximal complement targeting C3 and other proteins involved in the activation of the complement alternative pathway.8-10
Detection of residual complement activity in PNH patients on eculizumab, with their pathogenic and therapeutic implications. (A) Residual CH50 activity in PNH on eculizumab (see Figure 2A in the article by Peffault de Latour et al that begins on page 775). (B) Mechanism of residual intravascular hemolysis on eculizumab: lack of complete C5 blockade may be due to low plasma level of eculizumab (PK breakthrough) and extra complement activation (PD breakthrough) or rare C5 polymorphisms. Residual intravascular hemolysis may benefit from changes in eculizumab schedule and novel terminal complement inhibitors.10 (C) Mechanism of supervening extravascular hemolysis: due to the lack of CD55, proximal complement activation on RBC remains impaired, eventually leading to C3 opsonization and subsequent C3-mediated extravascular hemolysis.6 C3-mediated extravascular hemolysis may benefit from novel inhibitors of the proximal complement targeting C3 and other proteins involved in the activation of the complement alternative pathway.8-10
Impaired blood cell surface complement regulation is the hallmark of PNH, eventually accounting for its typical complement-mediated intravascular anemia.2 The anti-C5 monoclonal antibody eculizumab has drastically changed the landscape of PNH treatment; indeed, eculizumab has been proven effective in controlling intravascular hemolysis of PNH, resulting in the resolution of all hemolysis-related symptoms and in the reduction or even abolishment of the need for red blood cell (RBC) transfusions.3 Long-term treatment with eculizumab seems to reduce the risk of thromboembolic events,2,4 eventually leading to a significant improvement of survival.5 Nevertheless, the majority of PNH patients on eculizumab continue to exhibit mild to moderate anemia,2 and even patients achieving normal hemoglobin levels show increased reticulocyte counts, consistent with residual hemolysis.6 Thus, one may question how this is related to pharmacology of eculizumab. Available PD data on eculizumab are limited to the first 11 patients enrolled in the pilot study; in this study, the authors used a chicken RBC-based complement hemolytic assay, showing that full complement blockade was achieved in all patients with sustained pharmacologic levels of eculizumab.7 In the routine practice, no assay is available to monitor complement activity, and physicians may track therapeutic complement inhibition by using downstream biomarkers of hemolysis, such as lactate dehydrogenase (LDH), haptoglobin, bilirubin, and hemoglobinuria/hemosiderinuria.
Here Peffault de Latour et al report on a systematic PD study on 22 PNH patients on eculizumab, in which complement activity has been investigated by a standard hemolytic complement assay (CH50), in combination with free eculizumab dosage and other routine laboratory testing. The main finding of their study is that CH50 is a reliable biomarker of complement activity that may be used to track pharmacologic efficacy of complement inhibition. Furthermore, CH50 strongly correlates with free eculizumab levels, providing evidence that these 2 biomarkers are adequate for monitoring anticomplement treatment in PNH. Of the 22 patients who were longitudinally analyzed, incomplete C5 blockade was demonstrated in about half of the samples (see figure). Indeed, in the total of 364 samples, full blockade of CH50 was associated with lower LDH levels, showing that CH50 is a sensitive and instant marker of in vivo residual complement activity, which in PNH eventually results in detectable hemolysis. The correlation of CH50 with other laboratory measures of intravascular hemolysis (hemoglobin, reticulocytes, and bilirubin) was less evident than that with LDH (but few patients have been included), likely because the former are less instant markers of intravascular hemolysis (and they may also be affected by other conditions, such as extravascular hemolysis and marrow failure). CH50 correlates with free eculizumab levels; indeed, higher free eculizumab was associated with full CH50 blockade. However, free eculizumab level does not correlate with LDH, whereas it was associated with elevated bilirubin and need for RBC transfusions. These observations suggest that CH50 is the best PD measure to track instant complement blockade, whereas free eculizumab level represents the best pharmacokinetic (PK) measure to predict propensity to develop leaks in pharmacologic complement inhibition. Neither CH50 nor free eculizumab shows any correlation with C3 deposition, which was documented in all PNH patients on eculizumab, as previously reported.6
This study documents that PKs and PDs of eculizumab in PNH are not entirely predictable and eventually heterogeneous among patients; the actual clearance mechanism of both free and C5-bound eculizumab has not been described yet. Notably, whereas low circulating levels were obviously associated with lack of complement blockade, residual complement activity may be detected even in the presence of an excess of free eculizumab. This finding parallels the observation that pharmacologically effective levels of eculizumab are not able to fully prevent hemolysis in an in vitro extended acidified serum assay (which tests the functional activity of the alternative activating and terminal effector complement pathways).8,9 In this assay, even the in vitro addition of an excess of eculizumab does not revert this residual lytic complement activity (unpublished data). The reasons and the meaning of these observations remain to be elucidated, even if it is reasonable to think that they may reflect the incapability of eculizumab in facing an excess of complement activation as it may occur in vitro or in vivo. Indeed, hemolytic crises are commonly observed during infections, eventually as a consequence of an extra activation of the complement cascade; because these crises may occur irrespective of eculizumab levels, they should be described as a PD rather than PK breakthrough. It has to be remarked that an excellent clinical efficacy can be achieved irrespective of this residual complement activity, eventually raising the question whether the abolishment of this residual activity is a goal to be pursued for improving the results of current anticomplement treatment.
With their study, Peffault de Latour et al provide a reliable way to monitor therapeutic complement inhibition. Given that residual anemia in PNH patients on eculizumab may be due to different (but possibly concomitant) causes (residual intravascular hemolysis due to PK or PD breakthrough, bone marrow failure, C3-mediated extravascular hemolysis),10 these findings are useful to drive therapeutic decisions (see figure). Indeed, only PNH patients showing recurrent residual CH50 activity may benefit from modification of the treatment schedule (ie, increased doses or reduced administration intervals). This mechanistic demonstration of residual terminal complement activity is extremely interesting even in the context of the development of second-generation complement inhibitors. Indeed, a number of novel complement therapeutics are in preclinical and clinical investigations8-10 ; they may intercept complement at the level of its terminal effector components (eg, C5 inhibitors) or target the proximal activating complement. The latter include broad C3 inhibitors (eg, compstatin)9 and pathway-specific modulators of the initial complement activation (eg, alternative pathway inhibitors targeting complement factor B and factor I, or factor H-based engineered proteins).8 In the future scenario of a multioption complement inhibition, the assays proposed by Peffault de Latour et al will be pivotal to identify patients who may benefit from a second-line treatment with other terminal complement inhibitors and patients who better qualify for an upstream targeted intervention aiming to treat C3-mediated extravascular hemolysis (see figure).
Conflict-of-interest disclosure: A.M.R. has received research grants from Alexion Pharmaceuticals, Novartis, RApharma Pharmaceuticals, and Alnylam Pharmaceuticals.