Key Points
ACTN1 mutations were identified in 10 of 239 families with inherited thrombocytopenia of unknown origin.
ACTN1-related thrombocytopenia is characterized by mild thrombocytopenia with platelet macrocytosis and low risk for bleeding.
Abstract
Inherited thrombocytopenias (ITs) are a heterogeneous group of syndromic and nonsyndromic diseases caused by mutations affecting different genes. Alterations of ACTN1, the gene encoding for α-actinin 1, have recently been identified in a few families as being responsible for a mild form of IT (ACTN1-related thrombocytopenia; ACTN1-RT). To better characterize this disease, we screened ACTN1 in 128 probands and found 10 (8 novel) missense heterozygous variants in 11 families. Combining bioinformatics, segregation, and functional studies, we demonstrated that all but 1 amino acid substitution had deleterious effects. The clinical and laboratory findings of 31 affected individuals confirmed that ACTN1-RT is a mild macrothrombocytopenia with low risk for bleeding. Low reticulated platelet counts and only slightly increased serum thrombopoietin levels indicated that the latest phases of megakaryopoiesis were affected. Given its relatively high frequency in our cohort (4.2%), ACTN1-RT has to be taken into consideration in the differential diagnosis of ITs.
Introduction
Inherited thrombocytopenias (ITs) are a highly heterogeneous group of diseases characterized by different degrees of severity and complexity.1 The variable clinical phenotype derives from a wide genetic heterogeneity, with at least 22 genes having been identified so far.2-4 Moreover, mutations in known genes account for only 50% of patients, indicating that not all the existing forms have yet been identified. One of the most recently recognized disorders is ACTN1-related thrombocytopenia (ACTN1-RT), an autosomal -dominant form caused by mutations in the gene (ACTN1) encoding for 1 of the 2 nonmuscle isoforms of α-actinin 1.5 Mainly expressed in megakaryocytes and platelets,5 ACTN1 has a binding domain for cross-linking the actin filaments into bundles. Based on the 7 families described in the literature, ACTN1-RT is characterized by mild macrothrombocytopenia and bleeding tendency, and no additional defects associated with low platelet count.5,6 To better characterize ACTN1-RT, we studied 10 families identified after the screening of ACTN1 in 128 probands with an IT of unknown origin.
Research design and methods
Of 239 consecutive probands with IT examined at the Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo Foundation in Pavia, Italy, we enrolled all individuals (n = 128) without a certain diagnosis after the IT diagnostic work-up.7,8 Mutational screening of ACTN1 was carried out by whole-exome sequencing in 7 individuals and Sanger sequencing in the remaining 121 probands, as indicated in supplemental Table 1, available on the Blood Web site. Pathogenetic effects of variants were investigated by segregation analysis, bioinformatic tools, and immunofluorescence studies (supplemental Table 1; Figure 1; supplemental Figure 1). Medical history (family history included), bleeding tendency, and outcome of possible surgeries and pregnancies were ascertained from medical records or patient interviews. Research was conducted in accordance with the Declaration of Helsinki.
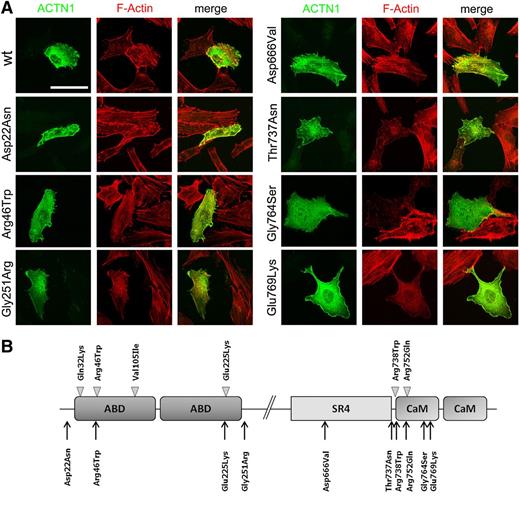
Functional studies of novel ACTN1 variants. (A) Immunofluorescence analysis in PD220 fibroblast cell line transiently transfected according to standard procedures. Both wild-type (wt) or mutant ACTN1 cDNAs were cloned into the pcDNA3.1-Myc tagged expression vector.5 The subcellular localization of exogenous α-actinin 1 (green) was examined using c-myc antibodies (9E10; Santa Cruz Biotechnology), whereas the actin filaments were stained with AlexaFluor594 (red) conjugated phalloidin (Invitrogen). Images were obtained with a Nikon C1si confocal microscope, using ×60 Plan Apo objectives. Images were processed for z-projection (maximum intensity), brightness, and contrast regulation, using ImageJ 1.45 (National Institutes of Health). The cells shown are representative of 3 independent experiments. Scale bar = 50 µm. (B) Domain structure of α-actinin and localization of ACTN1 mutations identified in Japanese families (arrowheads)5 and in this article (arrows). The p.Arg46Gln mutation was also identified in a French family.6 α-actinin is organized in an actin-binding domain (ABD) at the N terminus, 4 spectrin repeats, and a calmodulin-like domain (CaM) at the C terminus. Antiparallel molecules dimerize in rod-like structures with the ABD at each extremity for cross-linking the actin filaments into bundles.
Functional studies of novel ACTN1 variants. (A) Immunofluorescence analysis in PD220 fibroblast cell line transiently transfected according to standard procedures. Both wild-type (wt) or mutant ACTN1 cDNAs were cloned into the pcDNA3.1-Myc tagged expression vector.5 The subcellular localization of exogenous α-actinin 1 (green) was examined using c-myc antibodies (9E10; Santa Cruz Biotechnology), whereas the actin filaments were stained with AlexaFluor594 (red) conjugated phalloidin (Invitrogen). Images were obtained with a Nikon C1si confocal microscope, using ×60 Plan Apo objectives. Images were processed for z-projection (maximum intensity), brightness, and contrast regulation, using ImageJ 1.45 (National Institutes of Health). The cells shown are representative of 3 independent experiments. Scale bar = 50 µm. (B) Domain structure of α-actinin and localization of ACTN1 mutations identified in Japanese families (arrowheads)5 and in this article (arrows). The p.Arg46Gln mutation was also identified in a French family.6 α-actinin is organized in an actin-binding domain (ABD) at the N terminus, 4 spectrin repeats, and a calmodulin-like domain (CaM) at the C terminus. Antiparallel molecules dimerize in rod-like structures with the ABD at each extremity for cross-linking the actin filaments into bundles.
Results and discussion
Characterization of ACTN1 missense variants
Mutational screening of ACTN1 identified 10 different missense variants in 11 of 128 probands (supplemental Table 1). Whereas 2 (p.Arg738Trp and p.Arg752Gln) were known mutations,5 the others were novel variants not enlisted in the Single Nucleotide Polymorphism Database. Interestingly, c.136C>T (p.Arg46Trp) was found in 2 families and affected the same residue hit by the known c.137G>A (p.Arg46Gln) mutation.5,6 The 8 novel variants affected highly conserved amino acid residues (data not shown), and different pathogenicity prediction tools indicated deleterious effects. All but 1 (p.Asp666Val) of the variants segregated with thrombocytopenia within pedigrees when family members were available (supplemental Figure 1).
To determine the pathogenetic role of the novel missense variants, we performed immunofluorescence analysis in human fibroblasts (Figure 1A). Transfecting wild-type constructs, we observed that the cytoskeleton was organized in filaments, with ACTN1 colocalizing with actin. In cells expressing all but 1 (p.Asp666Val) of the mutations, ACTN1 was instead distributed uniformly within the cytoplasm, and actin was no longer organized in filaments. Consistent with segregation analysis, the correct organization of the cytoskeleton in cells expressing p.Asp666Val excluded this variant as a disease-causing mutation. It is worth noting that p.Asp666Val is outside the actin-binding and calmodulin-like domains (Figure 1B), suggesting that only alterations of these functional regions are compatible with the disease.
ACTN1-RT as a mild form of thrombocytopenia with slight platelet macrocytosis
Of the 10 families with pathogenetic variants, 9 were from Italy and 1 from the United Kingdom. Of the 31 affected family members, 22 were females and 9 males, indicating a potential diagnostic bias because of menorrhagia. At the time of recognition of thrombocytopenia (Table 19,10 ), the patients’ mean age was 34.5 years (range, 3-82 years). Mean platelet count was slightly reduced (103 ± 26 × 109/L), with only 1 patient having a platelet count lower than the cut-off value (50 × 109/L) for a safe platelet count.11 Of note, 2 young girls (families 5 and 8) had a platelet count slightly above 150 × 109/L, but they were considered thrombocytopenic according to the age- and sex-specific reference intervals in the Italian population.9 Both mean platelet volume (12.6 ± 1.7 fL; 95% confidence interval [CI], 12.0-13.2 fL) and diameter (3.2 ± 0.5 μm; 95% CI, 3.0-3.4 μm) were significantly higher in ACTN1-RT than in 50 control patients, with values of 10.1 ± 1.4 fL (95% CI, 9.7-10.4 fL) and 2.4 ± 0.3 μm (95% CI, 2.3-2.5 μm), respectively.
Features of families with ACTN1 mutations
| Family (number of individuals) . | Mean age at diagnosis, years (range) . | World Health Organization bleeding score* . | Mean platelet count,†,‡ ×109/L (range) . | Mean platelet volume,† fL (range) . | Mean platelet diameter,¶ μm (range) . | ACTN1 mutation . |
|---|---|---|---|---|---|---|
| F1 (4) | 43 (22-55) | 0 (1), 1 (3) | 107 (89-134) | 11.1 (10.1-12.0) | 2.8 (2.7-3.0) | p.Asp22Asn |
| F2 (4) | 46 (26-64) | 0 (1), 1 (2), 2 (1) | 103 (81-118) | 12.5 (10.6-14.7) | 3.3 (3.0-3.7) | p.Arg46Trp |
| F3 (4) | 42 (14-72) | 0 (1), 1 (1), 2 (2) | 95 (66-124) | 14.8 (14.0-15.6) | 3.8 (3.5-4.1) | p.Arg46Trp |
| F4 (2) | 30 (12-49) | 0 (2) | 103 (97-110) | 11.8 [11.3-12.4) | 3 (2.8-3.1) | p.Glu225Lys |
| F5 (6) | 23 (7-44) | 0 (1), 1 (3), 2 (3) | 103 (78-154) | 12.3 [10.4-14.0) | 3.3 (2.6-4.3] | p.Gly251Arg |
| F6 (2) | 58 (34-82) | 2 (2) | 58 (55-62) | 10.5 [10.4-10.6) | 3.2 (2.9-3.5] | p.Thr737Asn |
| F7 (1) | nd | 0 (1) | 110 | 12.1 | 2.5 | p.Arg738Trp |
| F8 (3) | 25 [3-44) | 0 (2), 1 (1) | 112 (65-166) | 12.5 (11.3-14.3) | 2.8 (2.6-3.0] | p.Arg752Gln |
| F9 (1) | 48 | 0 (1) | 117 | 14.4 | 3.3 | p.Gly764Ser |
| F10 (4) | 38 [3-66) | 0 (1), 1 (2), 2 (1) | 86 (46-120) | 14-3 [12.6-15) | 3.5 (2.9-3.9) | p.Glu769Lys |
| Family (number of individuals) . | Mean age at diagnosis, years (range) . | World Health Organization bleeding score* . | Mean platelet count,†,‡ ×109/L (range) . | Mean platelet volume,† fL (range) . | Mean platelet diameter,¶ μm (range) . | ACTN1 mutation . |
|---|---|---|---|---|---|---|
| F1 (4) | 43 (22-55) | 0 (1), 1 (3) | 107 (89-134) | 11.1 (10.1-12.0) | 2.8 (2.7-3.0) | p.Asp22Asn |
| F2 (4) | 46 (26-64) | 0 (1), 1 (2), 2 (1) | 103 (81-118) | 12.5 (10.6-14.7) | 3.3 (3.0-3.7) | p.Arg46Trp |
| F3 (4) | 42 (14-72) | 0 (1), 1 (1), 2 (2) | 95 (66-124) | 14.8 (14.0-15.6) | 3.8 (3.5-4.1) | p.Arg46Trp |
| F4 (2) | 30 (12-49) | 0 (2) | 103 (97-110) | 11.8 [11.3-12.4) | 3 (2.8-3.1) | p.Glu225Lys |
| F5 (6) | 23 (7-44) | 0 (1), 1 (3), 2 (3) | 103 (78-154) | 12.3 [10.4-14.0) | 3.3 (2.6-4.3] | p.Gly251Arg |
| F6 (2) | 58 (34-82) | 2 (2) | 58 (55-62) | 10.5 [10.4-10.6) | 3.2 (2.9-3.5] | p.Thr737Asn |
| F7 (1) | nd | 0 (1) | 110 | 12.1 | 2.5 | p.Arg738Trp |
| F8 (3) | 25 [3-44) | 0 (2), 1 (1) | 112 (65-166) | 12.5 (11.3-14.3) | 2.8 (2.6-3.0] | p.Arg752Gln |
| F9 (1) | 48 | 0 (1) | 117 | 14.4 | 3.3 | p.Gly764Ser |
| F10 (4) | 38 [3-66) | 0 (1), 1 (2), 2 (1) | 86 (46-120) | 14-3 [12.6-15) | 3.5 (2.9-3.9) | p.Glu769Lys |
nd, not determined.
World Health Organization bleeding score: grade 0, no bleeding; grade 1, only cutaneous bleeding; grade 2, mild blood loss; grade 3, gross blood loss, requiring transfusion; grade 4, debilitating blood loss, retinal or cerebral, associated with fatality.
Platelet count and mean platelet volume were measured with a Cell-Dyn Sapphire hematology analyzer (Abbott) in EDTA-anticoagulated blood samples within 1 hour from venipuncture.
One individual from family 5 and 1 person from family 8 with more than 150 × 109 platelets/L (bold) were classified as thrombocytopenic according to the recently proposed age- and sex-specific reference intervals.9
Mean platelet diameter was measured on peripheral blood films by optical microscopy and software-assisted image analysis (Axio-vision 4.5; Carl Zeiss), as previously reported.10 In brief, blood smears prepared with non-anticoagulated blood from fingerstick were stained with May-Grünwald-Giemsa staining, and the mean platelet diameter was calculated from 200 different cells.
The mean reticulated platelet count evaluated in 9 patients using a hematology analyzer was lower (1.16 ± 0.64 × 109/L; 95% CI, 0.48-2.07 per liter) than in 15 control patients (4.3 ± 0.96 × 109/L; 95% CI, 3.55-5.24 per liter), indicating that thrombocytopenia derived from reduced platelet formation. To further investigate this aspect, we measured the serum thrombopoietin (TPO) concentration, which is inversely related to the total megakaryocyte and platelet mass,12 with a commercially available ELISA kit (Quantikine Human TPO Immunoassay, R&D). The mean TPO level was slightly higher (22.3 ± 6.5 pg/mL; 95% CI, 13.1-31.5 pg/mL) in the 14 examined patients than in control patients (n = 40; 14.6 ± 10.8 pg/mL; 95% CI, 11.1-18.0 pg/mL), suggesting normal megakaryopoiesis, as revealed by bone marrow examination of the only patient who received this test.6 Both young platelet count and TPO levels indicate that thrombocytopenia derives from defects of the latest phases of megakaryocyte maturation. Consistent with this conclusion, investigations in mouse megakaryocytes showed that they had defective proplatelet formation and platelet release when expressing ACTN1 mutants.5
In vitro platelet aggregation in response to ADP, collagen, and ristocetin was normal, and the expression level of glycoproteins (GPs) GPIbα, GPIX, GPIIb, and GPIIIa on the platelet surface was increased, as expected in individuals with platelet macrocytosis (supplemental Table 2). Consistent with mild thrombocytopenia, as well as with platelet aggregation and expression of major platelet glycoproteins in the normal range, spontaneous bleeding tendency was absent or mild, with a few episodes of epistaxis, gum bleeding, easy bruising, and menorrhagia (Table 1). Only 3 females received iron therapy for menorrhagia. Four of the 18 patients undergoing surgery had excessive bleeding, but only 1 needed platelet transfusions. Of the 30 deliveries, only 1 had increased blood loss. Although limited, these severe hemorrhagic episodes suggest caution when hemostatic challenges occur.
Finally, we did not observe additional phenotypic defects consistently associated with ACTN1 mutations. However, it is worth mentioning that 3 individuals from family 2, whose genotypes and platelet counts were not available, developed leukemia (both age of onset and type of leukemia were not available) (supplemental Figure 1). Moreover, individuals from families 1 and 5 had mitral valve prolapse and/or atrial septal defect (supplemental Figure 1). Because 1 individual with atrial septal defect (family 5) did not carry the mutation (p.Gly251Arg) segregating with thrombocytopenia in his family, a gene other than ACTN1 is likely to be responsible at least for this heart malformation. Because the search for cardiac anomalies was not systematically performed in our case series, we cannot exclude that other patients with ACTN1-RT had similar defects. Hence, echocardiography may be appropriate in this form of IT.
ACTN1-RT is relatively common among the different forms of IT, at least in Italy and Japan.5 Indeed, we identified ACTN1 mutations in 10 (4.2%) of 239 consecutive IT families, a frequency similar to that reported in the Japanese population (3.7%).5 Considering only the ITs with large platelets, the frequency is 6.6% in our population. However, we cannot exclude that ACTN1-RT is more frequent than expected, as affected subjects have no pathognomonic features and may be easily misdiagnosed as having immune thrombocytopenia.13 Four of our patients had this diagnosis, and 3 were treated with steroids without any effect on platelet count. Therefore, molecular genetic testing, combined with segregation analysis and functional assays carried out to discriminate between pathogenic and silent variants, is the only diagnostic approach to identify patients with ACTN1-RT.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Professor Kerry J. Rhoden for his critical review of the manuscript.
This work was supported by grants GGP13082 from Telethon Foundation (P.N., A.S.) and 15/12 and 16/12 (Ricerca Corrente) from Istituto di Ricovero e Cura a Carattere Scientifico Burlo Garofolo (A.S.).
Authorship
Contribution: R.B., C.M., and T.P. carried out mutational screening and analyzed data; M.F. and G.B. performed immunofluorescence analysis and analyzed data; S.K. generated vectors; A.P., C.C., U.R., S.P., L.N., C.B., and P.N. enrolled patients, provided biological samples and analyzed clinical data; and C.L.B., M.S., A.S., and P.N. designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anna Savoia, Department of Medical Sciences, University of Trieste, IRCCS Burlo Garofolo, Via dell’Istria 65/1, 34137 Trieste; e-mail: anna.savoia@burlo.trieste.it.
References
Author notes
R.B., C.M., and M.F. contributed equally to this study.