Abstract
HIV-associated classical Hodgkin lymphoma (HIV-cHL) is an important complication of HIV disease in the era of effective combination antiretroviral therapy (cART). Generally, newly diagnosed HIV-cHL should be managed with curative intent. With modern HIV therapeutics, HIV-cHL treatment outcomes are largely comparable to those of the background population with cHL (non–HIV-cHL). To achieve these outcomes, particular attention must be given to managing HIV. This management includes understanding HIV as a comorbid condition with a spectrum of impact that is unique to each patient. Meticulous attention to drug-drug interactions is required to avoid toxicity and pharmacokinetic effects that can undermine cure. Relapsed and refractory HIV-cHL poses additional therapeutic challenges. The standard management in this setting should also be based on that for non–HIV-cHL, and includes the use of salvage chemotherapy followed by autologous stem cell transplant in chemosensitive disease. The role of allogeneic hematopoietic stem cell transplant is less clear but may be useful in select cases. Newer agents with activity in cHL are being tested as part of primary and salvage therapy and are also highly relevant for HIV-cHL.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1355.
Disclosures
The authors, Associate Editor Jacob M. Rowe, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the epidemiology of human immunodeficiency virus (HIV)-associated classical Hodgkin lymphoma, based on a review.
Distinguish the clinical presentation and diagnosis of HIV-associated classical Hodgkin lymphoma.
Discuss the clinical management of HIV-associated classical Hodgkin lymphoma.
Release date: February 19, 2015; Expiration date: February 19, 2016
Introduction
HIV-associated classical Hodgkin lymphoma (HIV-cHL) is a serious complication of HIV. As with Kaposi sarcoma and non-Hodgkin lymphomas (NHLs), cHL risk is substantially elevated by HIV infection. However, unlike those tumors, HIV-cHL does not confer a diagnosis of AIDS. HIV-cHL may present with extranodal involvement that can lead to life-threatening organ dysfunction. Nonetheless, outcomes comparable to those seen in the general population are possible with standard curative-intent therapy and modern combination antiretroviral therapy (cART). In this article, we discuss the epidemiology, pathobiology, and clinical management of HIV-cHL.
Case presentation
A 44-year-old man was referred with relapsed HIV-cHL. He initially presented 20 months prior with supraclavicular swelling, night sweats, and weight loss. Excisional lymph node biopsy demonstrated Epstein-Barr virus (EBV)-associated cHL, mixed cellularity (MC) subtype (Figure 1). HIV serology revealed the previously undiagnosed infection. CD4+ T-cell count (CD4+ count) was 140 cells per mm3. He commenced tenofovir, lamivudine, and ritonavir-boosted atazanavir for HIV, as well as trimethoprim/sulfamethoxazole for Pneumocystis pneumonia prophylaxis. He had no history of opportunistic infections (OIs). 18Fluorodeoxyglucose (18FDG) positron emission tomography (PET) revealed 18FDG uptake in lymph nodes and bones (Figure 2A). He had advanced-stage, unfavorable disease (stage IVB and International Prognosis Score of 4 based on gender, stage, hemoglobin level of 10 mg/dL, and albumin level of 2.0 mg/dL). He received doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) with pegfilgrastim and continued cART. His first cycle was complicated by grade 4 neutropenic fever lasting 2 weeks. During subsequent cycles, vinblastine and dacarbazine were dose reduced by 50%. Additionally, there were dose delays during several cycles due to neutropenia, and the patient developed grade 2 neuropathy. 18FDG-PET after 2 cycles showed uptake in the left axilla. A biopsy sample taken in response to a solitary radiographic lymph node abnormality after 6 28-day cycles showed reactive changes. He completed 8 cycles and achieved a complete response (CR). After therapy, CD4+ count was 173 cells per mm3.
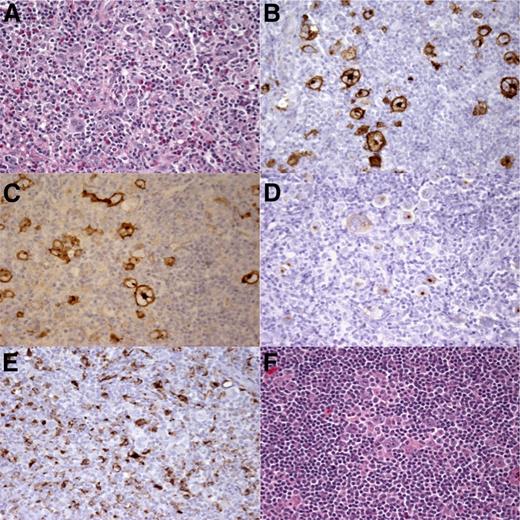
Histopathology and immunohistochemistry of HIV-cHL. (A) Hematoxylin and eosin staining shows cHL, MC subtype. Immunostaining for (B) CD15, (C) CD30, and (D) EBV latent membrane protein 1 demonstrates Hodgkin Reed-Sternberg cells. (E) CD68 staining showing many (>5%)46 macrophages. (F) Hematoxylin and eosin staining at time of relapse shows cHL, MC subtype. Original magnification ×40.
Histopathology and immunohistochemistry of HIV-cHL. (A) Hematoxylin and eosin staining shows cHL, MC subtype. Immunostaining for (B) CD15, (C) CD30, and (D) EBV latent membrane protein 1 demonstrates Hodgkin Reed-Sternberg cells. (E) CD68 staining showing many (>5%)46 macrophages. (F) Hematoxylin and eosin staining at time of relapse shows cHL, MC subtype. Original magnification ×40.
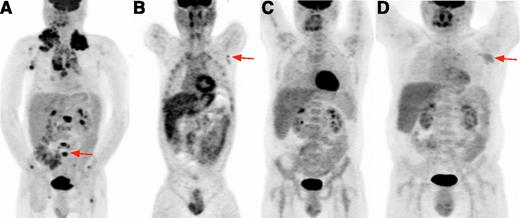
18FDG-PET in HIV-cHL. (A) Baseline 18FDG-PET. Volumetric image shows bulky intensely hypermetabolic cervical, mediastinal, and axillary lymph nodes and multiple focal bone lesions (representative vertebral lesion, red arrow). (B) Interim 18FDG-PET. At the end of cycle 2, coronal image focuses on a small suspicious lesion in left axilla (red arrow); diffuse bone uptake attributable to pegfilgrastim is also noted. After cycle 6, a biopsy sample of residual abnormalities in the left axilla showed reactive changes and no evidence of cHL. (C) End-of-therapy 18FDG-PET. Volumetric image shows resolution of 18FDG avid nodes. (D) Relapse 18FDG-PET. Volumetric image shows left axillary avid lymph node (red arrow) and other small nodes above the diaphragm.
18FDG-PET in HIV-cHL. (A) Baseline 18FDG-PET. Volumetric image shows bulky intensely hypermetabolic cervical, mediastinal, and axillary lymph nodes and multiple focal bone lesions (representative vertebral lesion, red arrow). (B) Interim 18FDG-PET. At the end of cycle 2, coronal image focuses on a small suspicious lesion in left axilla (red arrow); diffuse bone uptake attributable to pegfilgrastim is also noted. After cycle 6, a biopsy sample of residual abnormalities in the left axilla showed reactive changes and no evidence of cHL. (C) End-of-therapy 18FDG-PET. Volumetric image shows resolution of 18FDG avid nodes. (D) Relapse 18FDG-PET. Volumetric image shows left axillary avid lymph node (red arrow) and other small nodes above the diaphragm.
Twelve months after completing chemotherapy, a new axillary lymph node was palpated. Computerized tomography (CT) revealed a 2.5-cm axillary lymph node and other new, smaller lymph nodes. Excisional biopsy revealed recurrent EBV-positive (EBV+) cHL lymphoma, MC subtype. CD4+ count was 145 cells per mm3 and HIV viral load remained undetectable. 18FDG-PET revealed no extranodal disease (Figure 2D). We reasoned that drug-drug interactions between HIV protease inhibitor (PI)-based therapy and vinblastine complicated his initial treatment, and that although he had recurrent disease at 12 months, it was likely chemosensitive owing to initial response despite prior dose reductions and cycle delays. Therefore, to reduce drug-drug interactions, we modified his cART, replacing ritonavir and atazanavir with efavirenz. Given his prior intolerance to therapy, we elected to employ dose-adjusted etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone, and filgrastim rather than conventional cHL salvage. We anticipated that vincristine would be less affected by efavirenz than would be the case with vinblastine, and that dose intensity could be adjusted based on the prior cycle. Due to the low-dose prolonged infusions, we anticipated minimal cardiac toxicity from additional doxorubicin. After 2 cycles, CT showed no disease, and he proceeded to autologous stem cell transplant (auto-SCT) following carmustine, etoposide, cytarabine, and melphalan conditioning. CD4+ count 3 months posttransplant was 186 cells per mm3. The patient has been followed on cART for 7 years with no relapse, undetectable HIV with CD4+ count rising to >500 cells per mm3, and resolution of his neuropathy.
Epidemiology
cHL in patients with HIV
cHL incidence is approximately 50 per 100 000 person-years among HIV-infected individuals, fivefold to 20-fold higher than in the background population.1-4 Nodular lymphocyte–predominant HL does not have an established association with HIV, although cases in HIV-infected people have been noted.3 Unlike HIV-associated NHL (HIV-NHL), in which incidence decreased by 50% after introduction of cART (mainly owing to decreases in immunoblastic histologic types),5,6 HIV-cHL risk has remained stable and burden of disease has increased.2,6,7 In the United States, cHL follows NHL, Kaposi sarcoma, lung cancer, and anal cancer as the fifth commonest tumor in the setting of HIV.6 The median age of 40 to 44 years, which after correcting for age distribution of the at-risk population, is older than that of the general population.8 Indeed, in the United States, 14% of the cHL cases in this age group are HIV associated. Between 2000 and 2010, a large proportion of cHL in African Americans (17%) and Hispanics (10%) was attributed to HIV,3 reflecting shifting demographics of the HIV epidemic in the United States. In Johannesburg, South Africa, where HIV seroprevalence is 20% in young adults, 60% of cHL cases are HIV related.9 As with NHL,10-13 over time there has been a pathobiological shift in cHL subtypes in patients with HIV. Prior to cART, MC was the commonest subtype.14-17 In the cART era, likely due to improved immunity among HIV-infected populations,2 the nodular sclerosis subtype now accounts for nearly 50% of cases.3
CD4+ counts, initiation of cART, and HIV-cHL
The association between HIV-cHL and CD4+ count is complex. Median CD4+ count at cHL diagnosis is between 150 and 250 cells per mm3; however, HIV-cHL risk is elevated at all CD4+ counts.2,4,13 Additionally, besides being a manifestation of untreated HIV, lymphocytopenia is also a common manifestation of cHL.18 In case-control studies of HIV patients matched for clinical features including time on cART, the decrease in median CD4+ count for those who developed HIV-cHL was 100 cells per mm3 over 12 months prior to cHL diagnosis compared to a median increase of 35 cells per mm3 in controls.4,19 Indeed, a precipitous drop in CD4+ count may be a heralding event of HIV-cHL.4,20
The period of highest risk of HIV-cHL diagnosis is in the months after initiating cART.19-22 Data from a large US Department of Veterans Affairs cohort suggest that the incidence may be as high as 250 per 100 000 person-years during the first year of cART, with a slow decline over 10 years with controlled HIV, and a substantially lower risk thereafter.21,23 It has been speculated that a certain amount of immune competence is required for cHL to develop. Alternatively, with cART initiation and control of HIV viremia, an inflammatory condition may occur that promotes or unmasks existing cHL.24 It is not possible to identify those destined to develop HIV-cHL on the basis of CD4+ dynamics alone.23 Vigilance for inflammatory, infectious, and malignant etiologies of immune reconstitution syndromes in all HIV patients initiating cART is recommended. Prompt evaluation may lead to improved outcomes.
Pathobiology
Hodgkin Reed-Sternberg (HRS) cells, which make up a minority of tumor cells in cHL, are clonal, mature B cells that express activation markers CD15 and CD30.25-28 Distinct histologic patterns in the inflammatory background form the basis for cHL subcategorization. In HIV-cHL, HRS cells are usually EBV+. These EBV-infected HRS cells exhibit a type II latency pattern in which expression of EBV-encoded genes is largely limited to Epstein-Barr nuclear antigen 1 and latent membrane proteins (LMP1, LMP2A, LMP2B). By in situ staining for EBV-encoded small RNA, up to 80% of nodular sclerosis and MC HIV-cHL cases are EBV-associated compared with <40% of non–HIV-cHL.16,26,29 EBV-encoded genes modulate cellular signaling pathways and contribute to pathogenesis. For example, LMP125,28,30,31 is a constitutively active homolog of CD40 that upregulates nuclear factor-kappa B signaling and is essential for B-cell transformation in vitro,32 whereas LMP2A mimics B-cell receptor signaling and also contributes to proliferation.33 Epstein-Barr nuclear antigen 1 appears to influence cytokine networks by stimulating the production of chemokines such as CXCL10 and CCL20,34,35 thus attracting T-regulatory cells and inhibiting an immune response against the EBV+ cells.
Gene expression profiling has informed the pathogenetic role of the tumor microenvironment in EBV+ cHL.36,37 Furthermore, EBV+ cHL tends to group with high-risk gene expression profiles in cHL. Although EBV infection of HRS cells is not the only factor leading to a high-risk molecular signature,38 modulation by EBV of host cellular pathways may promote high-risk features that typify the clinical presentation. Other unique features in HIV-cHL (compared with non–HIV-cHL) include decreased nodal CD4+ T-cells and lack of CD4+ rosetting around HRS cells, which may parallel low circulating CD4+ counts. CD8+ cell infiltrates are preserved; however, cytotoxic granzyme B expression is decreased compared with non–HIV-cHL,25,28,39-42 suggesting a role for defective EBV-specific immunity in disease pathogenesis.43 HIV itself may influence the nodal microenvironment and the CD4+ T-cell fluctuations after cART initiation, possibly contributing to HIV-cHL pathogenesis. Recruitment of reconstituted CCR4+CD4+ T cells by HRS secretion of CCL17 is a provocative possibility, but as yet, the associations with EBV-infected HRS remain unclear.44,45
Tumor infiltrating macrophages (TAMs), recruited in association with CD115 expression by HRS cells,37 are increasingly recognized as important in the pathophysiology of cHL.46-48 TAMs are prominent in EBV-associated cHL,47,48 including HIV-associated cases. Interestingly, the number of TAMs in HIV-cHL does not appear to differ significantly with cART,39 which, among other factors, may help explain why HIV-cHL risk does not appear to be substantially reduced by cART.42
Management
Clinical presentation, diagnosis, and staging
HIV-cHL often presents with advanced disease. Extranodal sites of disease, including bone marrow, liver, and spleen, as well as B symptoms are more common compared with the background population16,49 and can occur in the absence of enlarged lymph nodes. Diagnosis is established with biopsy samples of involved tissue, usually a lymph node. HRS cells are identified by morphologic characteristics, and immunostaining demonstrates them to be CD15+, CD30+. PAX5-positive and EBV-encoded small RNA–positive HRS cells support the diagnosis.
Clinical evaluation includes a history and physical, complete blood count with differential, albumin level, liver and kidney function, baseline pulmonary function test for patients undergoing bleomycin-containing regimens, and 18FDG-PET with integrated or concurrent CT of the neck, chest, abdomen, and pelvis.50 18FDG-PET must be interpreted with caution because HIV viremia and certain OIs can cause 18FDG-avid nodes.51-53 Although not validated in the setting of HIV-cHL, 18FDG-PET is both sensitive and specific for detecting bone marrow involvement in cHL. Revised cHL staging criteria recommend omitting the bone marrow biopsy in non–HIV-cHL,54 and we recommend omitting it in most HIV-related cases as well. Nonetheless, bone marrow biopsy may be diagnostically useful in patients with cytopenias, because employing Occam’s razor to unify the clinical findings within a single diagnosis can lead to missed diagnoses in HIV-infected patients. For example, reactive hemophagocytic lymphohistiocytosis in HIV-infected patients can be attributed to HIV-cHL in up to 20% of cases,55 and bone marrow biopsy in this setting can provide important information about the etiology of cytopenias. In the absence of specific risk stratification for HIV-cHL, patients should be classified as having early favorable, early unfavorable, or advanced disease using criteria for non–HIV-cHL.
Evaluation of HIV status and comorbidities
Given the high proportion of cHL attributed to HIV, we recommend asking all adults with cHL for permission to test for HIV during the initial clinical evaluation. Treating cHL in a patient with unrecognized HIV infection introduces undue risk, including treatment-related death, whereas treating cHL and known HIV allows for optimal management of both diseases.
Comorbid HIV has a broad spectrum of severity that requires evaluation during initial treatment planning. CD4+ count, HIV viral load, and, in some cases, HIV genotyping are integral to this assessment. Additional HIV-specific medical history includes duration of infection, HIV treatment history and response to therapy as measured by CD4+ count and HIV viral load, and complications of HIV or its therapy. The state of health prior to cancer development provides critical information. At the healthy end, with high CD4+ count and undetectable virus, HIV may be nearly inconsequential for initial cHL management. At the opposite end, where there is HIV-drug resistance and advanced CD4+ T-cell depletion, severe AIDS complications may render standard chemotherapy inadvisable. With improvements in HIV medicine, the latter scenario is increasingly rare, and it is essential that this determination be based on expert knowledge.
Although many patients will have undetectable HIV viral loads when on cART, some will have HIV viremia resulting from either ineffective therapy or no therapy. Patients continue to be diagnosed with HIV/AIDS after presenting late to medical attention for a variety of reasons, including lack of access to care, social stigma, and fear. HIV diagnosis made during a malignancy evaluation often presents an opportunity to successfully engage such patients in medical care, as illustrated in the case presentation. Treatment-experienced patients may have uncontrolled HIV viremia for several reasons. HIV mutations, especially in patients with a complex history of cART use, may confer resistance to one or more antiretroviral agents. These are sometimes found in de novo infection as well. HIV resistance affects short-term and long-term management and requires expert evaluation including HIV genotyping.56 Lack of viral suppression due to cART nonadherence is important to recognize and address.
We evaluate degree of immunosuppression on the basis of CD4+ count, history of OIs, and CD4+ count nadir. Patients with CD4+ counts >350 cells per mm3 are less likely to have HIV-specific complications during cHL therapy; those with CD4+ counts between 100 and 350 cells per mm3 have increased risk that can be mitigated with antimicrobial prophylaxis; and those with CD4+ counts <100 per mm3 are at highest risk and require additional prophylaxis throughout treatment and follow-up (Table 1). A history of prior OIs or a low CD4+ count nadir may indicate a more vulnerable immune status than reflected in contemporary CD4+ counts. We employ regular surveillance for treatment-related events (ie, infections, mucositis) and have a low threshold to initiate evaluation for infectious complications in all HIV-infected patients, particularly those with prior OIs or a current or nadir CD4+ count of <200 cells per mm3. We evaluate CD4+ counts each cycle, then every 3 months for the first year following chemotherapy.
Suggested OI prophylaxis in patients with HIV and cHL undergoing chemotherapy
| OI . | Threshold for prophylaxis . | Prophylactic regimens . |
|---|---|---|
| Pneumocystis jirovecii pneumonia | All patients during chemotherapy; continue after therapy until a CD4+ count >200 cells per mm3 is sustained for 3-6 mo. | Preferred |
| Trimethoprim/sulfamethoxazole, 800/160-mg tablet (Bactrim DS) Monday, Wednesday, and Friday | ||
| Trimethoprim/sulfamethoxazole, 400/80-mg tablet daily | ||
| Alternatives | ||
| Pentamadine, 300 mg in 6 mL of sterile water via nebulizer inhaler, once monthly | ||
| Atovaquone, 1500-mg suspension once daily | ||
| Dapsone, 100-mg tablet daily; mild hemolysis may be seen in patients with glucose-6-phosphate dehydrogenase deficiency | ||
| Trimethoprim/sulfamethoxazole and atovaquone may also prevent toxoplasmosis. | ||
| Herpes simplex virus 1/2 | All patients with history of oral or anogenital herpes simplex virus. Follow general recommendations for secondary prophylaxis starting 3-6 mo after completion of therapy. | Valacyclovir, 1000-mg tablet daily |
| Famciclovir, 500-mg tablet twice daily | ||
| Acyclovir, 400-mg tablet twice daily | ||
| These agents may also provide prophylaxis against varicella-zoster virus reactivation. | ||
| Candida albicans (especially oral thrush) | Consider primary prophylaxis when CD4+ count is <100 cells per mm3, or secondary prophylaxis with history of mucosal candidiasis. Alternatively, can be treated on earliest symptoms, especially when CD4+ count is >200 cells per mm3. | Fluconazole, 200-mg tablet once daily (or 3 times/wk); also prevents cryptococcal disease in HIV patients with CD4+ counts <100 cells per mm3; do not administer the day before or the day of chemotherapy |
| Nystatin oral suspension, 5 mL “swish and swallow” 2-4 times daily (may be included in oral mouthwashes used for management of mucositis) | ||
| Atypical mycobacteria (ie, Mycobacterium avium complex or Mycobacterium intracellulare) | CD4+ count <100 cells per mm3. Consider a higher current CD4+ threshold in patients with recent history of a nadir <100 cells per mm3 or history of atypical mycobacterial infection. | Azithromycin, 1200 mg weekly |
| OI . | Threshold for prophylaxis . | Prophylactic regimens . |
|---|---|---|
| Pneumocystis jirovecii pneumonia | All patients during chemotherapy; continue after therapy until a CD4+ count >200 cells per mm3 is sustained for 3-6 mo. | Preferred |
| Trimethoprim/sulfamethoxazole, 800/160-mg tablet (Bactrim DS) Monday, Wednesday, and Friday | ||
| Trimethoprim/sulfamethoxazole, 400/80-mg tablet daily | ||
| Alternatives | ||
| Pentamadine, 300 mg in 6 mL of sterile water via nebulizer inhaler, once monthly | ||
| Atovaquone, 1500-mg suspension once daily | ||
| Dapsone, 100-mg tablet daily; mild hemolysis may be seen in patients with glucose-6-phosphate dehydrogenase deficiency | ||
| Trimethoprim/sulfamethoxazole and atovaquone may also prevent toxoplasmosis. | ||
| Herpes simplex virus 1/2 | All patients with history of oral or anogenital herpes simplex virus. Follow general recommendations for secondary prophylaxis starting 3-6 mo after completion of therapy. | Valacyclovir, 1000-mg tablet daily |
| Famciclovir, 500-mg tablet twice daily | ||
| Acyclovir, 400-mg tablet twice daily | ||
| These agents may also provide prophylaxis against varicella-zoster virus reactivation. | ||
| Candida albicans (especially oral thrush) | Consider primary prophylaxis when CD4+ count is <100 cells per mm3, or secondary prophylaxis with history of mucosal candidiasis. Alternatively, can be treated on earliest symptoms, especially when CD4+ count is >200 cells per mm3. | Fluconazole, 200-mg tablet once daily (or 3 times/wk); also prevents cryptococcal disease in HIV patients with CD4+ counts <100 cells per mm3; do not administer the day before or the day of chemotherapy |
| Nystatin oral suspension, 5 mL “swish and swallow” 2-4 times daily (may be included in oral mouthwashes used for management of mucositis) | ||
| Atypical mycobacteria (ie, Mycobacterium avium complex or Mycobacterium intracellulare) | CD4+ count <100 cells per mm3. Consider a higher current CD4+ threshold in patients with recent history of a nadir <100 cells per mm3 or history of atypical mycobacterial infection. | Azithromycin, 1200 mg weekly |
We screen for hepatitis B virus (HBV) and hepatitis C virus (HCV) with serology for hepatitis B surface antigen, antibody to hepatitis B core, antibody to hepatitis B surface antigen, and antibody to HCV. Patients with a positive hepatitis B surface antigen require an HBV viral load and treatment of HBV. We monitor persons who test positive for antibody to hepatitis B core closely for signs of liver disease. For positive HCV serology, we evaluate HCV RNA viral load. Sensitivity of HCV serology is suboptimal in HIV-infected patients, with 13% of seronegative patients having evidence of HCV on RNA testing. Therefore, HCV RNA testing is also advised in HCV-seronegative patients with a history of intravenous drug use, aspartate aminotransferase/alanine aminotransferase abnormalities, and/or thrombocytopenia.57 Given the advances in curative HCV therapy, we refer HCV-infected patients for treatment after completion of chemotherapy.
Primary chemotherapy for HIV-cHL
Historically, absent cART, long-term HIV-cHL outcomes were poor. As with HIV-NHL, low-dose chemotherapy strategies were sometimes used58,59 ; however, this method did not improve survival, and low-dose chemotherapy approaches are no longer appropriate. Adherence to chemotherapy dose and schedule in cHL therapy, even in the face of neutropenia (nonfebrile), is critical for optimizing the probability of cure for both non–HIV-cHL60 and HIV-cHL.61
High cure rates in HIV-cHL in the cART era have recently been demonstrated. The German HIV-related Lymphoma Study Group prospectively evaluated a stage-adapted approach. Patients with early-stage favorable disease received 2 to 4 cycles of ABVD and involved-field radiation; patents with early unfavorable or advanced disease were largely treated with 6 to 8 cycles of standard BEACOPP (bleomycin, etoposide, cyclophosphamide, vincristine, procarbazine, and prednisone). Most received concurrent cART. The CR rates for patients with early favorable, early unfavorable, and advanced HIV-cHL were 96%, 100%, and 86%, respectively. The 2-year disease-free survival rate was 95% for early-stage disease and 89% for advanced-stage disease. The 2-year overall survival (OS) rate in the entire cohort was 90.7%.62 However, BEACCOPP was toxic; dose reductions and delays were common, and treatment-related mortality was 7%. Our approach is to avoid BEACOPP in HIV-cHL, with the possible exception of response-adapted therapeutic escalation. This approach is consistent with treatment practice for non–HIV-cHL in the United States, where BEACOPP is generally viewed as more likely to contribute to late treatment-related myelodysplastic syndrome or leukemia. Likewise, concerns for late radiotherapy effects, such as secondary cancers and cardiovascular disease, are also important for HIV-infected patients, a population potentially more vulnerable to these effects.
Drug-drug interactions must be taken into consideration when managing HIV-cHL. In a retrospective analysis, 2 Spanish study groups found potentially life-threatening adverse events with coadministration of the HIV-PI ritonavir, the backbone of many commonly used PI-based cART regimens. Of 61 patients treated with ABVD and ritonavir-based cART, 10% died of treatment-related infections and 41% required dose delays.63 Subsequently, ritonavir, through strong CYP3A4 inhibition, has been demonstrated to exert pharmacokinetic effects on vinblastine resulting in severe neutropenia and neuropathy.64,65 In contrast, a recent retrospective comparison of 93 HIV-infected and 131 HIV-unrelated patients treated with ABVD from 1997 to 2010 found similar outcomes in the 2 populations. What distinguishes this report from the previous is that 92% of HIV-infected patients received a PI-sparing cART regimen. Both groups received comparable cHL treatment. HIV-infected patients also received appropriate OI prophylaxis. Only 1 toxic death was noted among HIV-infected patents. Five-year disease-free survival and OS rates in HIV-infected vs uninfected patients, respectively, were 85% vs 87% and 81% vs 88%, with no statistically significant differences between groups. HIV was not a predictor for OS after correcting for International Prognosis Score.59
Taken together, we currently recommend treating HIV-cHL as one would treat non–HIV-cHL, with careful management of HIV. The optimal care for the patient with HIV-cHL should be guided by current approaches for early favorable, early unfavorable, and advanced disease in the non-HIV setting. Accordingly, in the United States, ABVD remains the general standard-of-care first-line therapy for both early and advanced non–HIV-cHL, and existing evidence, although limited, generally supports ABVD as a standard in HIV-cHL.66,67 For bulky stage I and II disease in non–HIV-cHL, radiotherapy is commonly employed; however, there is interest in reducing or eliminating radiotherapy for at least some of these patients,68 and this treatment approach is the focus of ongoing National Cancer Institute (NCI)-sponsored trials. In our opinion, granulocyte colony-stimulating factors,69 especially for patients with CD4+ counts <200 cells per mm3, should be used more liberally than in HIV-unrelated cases for which primary neutropenic fever prophylaxis is not recommended.
Approach to cART and supportive care
We either continue cART or initiate cART as early as possible when managing patients with HIV-cHL. This practice is informed by ex-perience, because there are no prospective data to inform optimal timing of cART initiation in this setting. Available data raise the possibility of inadequate CR rates when concurrent cART is not employed.59,70-72 Nonetheless, when the only option is to use cART that negatively impacts the ability to administer curative-intent chemotherapy, we will defer cART until completion of treatment of HIV-cHL based on experience in treating HIV-NHL.12,73 With the increasing availability of antiretroviral agents and the option of several cHL regimens, this tactic has become increasingly unnecessary. In the setting of hepatic or renal dysfunction, we consider temporarily suspending or deferring cART to minimize polypharmacy. Drug-drug interactions may be particularly complicated in patients with low CD4+ counts and concurrent infections requiring treatment or in those with limited cART options.
For symptomatic patients with advanced HIV-cHL, starting chemotherapy as soon as possible is usually desired and often leads to improvement in both performance status and tumor-associated organ dysfunction. An appropriate cART regimen can be started after initial administration of ABVD. Occasionally, unanticipated adverse events occur when administering chemotherapy and concurrent cART. In such cases, we prioritize cHL in treatment decisions and avoid chemotherapy delays. If necessary, cART can be suspended. After chemotherapy, we may modify cART on the basis of patient and HIV-related factors. In addition to managing HIV, monitoring for OIs (especially oral candidiasis) and select use of OI prophylaxis (Table 1) are required in treating HIV-cHL. Optimal outcomes are fostered by collaborative teams with expertise in infectious disease, pharmacology, social support, and patient education. Additional specialists may be required.
A basic principle we follow is to eliminate, where possible, concomitant medications (including antiretroviral drugs) that constrain the ability to administer full-dose and on-schedule chemotherapy for cHL. Facilitating this approach are more than 35 US Food and Drug Administration–approved antiretroviral drugs and combined-pill combinations (Table 2). Regimens generally include a 3-drug combination of 2 NRTIs and 1 drug from another class. We avoid older NRTIs due to their excess toxicity and believe that ritonavir, used alone or as a pharmacologic booster, and other strong CYP3A4 inhibitors such as cobicistat are essentially contraindicated for coadministration during ABVD. We also avoid other PIs due to CYP3A4-inhibitory properties.61 Preferred cART regimens have minimal expected CYP3A4 effects and employ agents with low stand-alone toxicity. At present, a regimen of an integrase strand transfer inhibitor (dolutegravir or raltegravir) or certain nonnucleoside reverse transcriptase inhibitors (rilpivirine) along with an NRTI combination of emtricitabine and tenofovir (Truvada) or abacavir and lamivudine (Epzicom), is likely to have minimal clinically relevant adverse effects on cancer therapy for HIV-cHL. Additional regimens are feasible but require consideration of agents or combinations not listed in Table 2. In patients treated with cART, adherence should be assessed each cycle. We use a simple 7-day recall tool74,75 that entails asking the number of missed cART doses over the last 7 days. For those reporting less than 100% adherence, evaluation of the underlying reasons for nonadherence is required. We recommend HIV viral-load monitoring at each cycle until viral suppression is documented in patients initiating or changing cART and in patients with possible nonadherence.
Select antiretroviral therapy agents with level AI evidence for treating HIV, potential CYP3A4 interactions, and other considerations for concurrent use with chemotherapy
| Class/drugs . | Dose . | CYP3A4 interactions . | Side effects . | Other considerations . |
|---|---|---|---|---|
| First-choice agents* | ||||
| NRTIs | ||||
| Emtricitabine/tenofovir (Truvada) | 1 tablet (200 mg/300 mg) daily | — | Nephrotoxicity (<3%) | Dose adjust for creatinine clearance <50 mL/min |
| Dual anti-HBV activity, preferred in HBV coinfected patients | ||||
| AI† NRTI in several NNRTI-, PI-, and INSTI-based regimens | ||||
| Abacavir/lamivudine (Epzicom) | 1 tablet (600 mg/300 mg) daily | — | Life-threatening hypersensitivity reactions in patients with HLA-B57*01 allele | Pharmacogenomic testing for HLA-B57*01 required before use of abacavir |
| Avoid Epzicom if creatinine clearance <50 mL/min; instead, use renally dosed individual agents | ||||
| AI† NRTI in combination with dolutegravir | ||||
| INSTIs | ||||
| Dolutegravir (Tivicay) | 1 tablet (50 mg) daily | — | Elevated AST/ALT | Metabolized by UGT1A1; dose increase required with efavirenz, rifampin, or select ritonavir-based combinations, and possibly other UGT1A1 inducers |
| Increase dose to 50 mg twice daily for patients with certain INSTI-related mutations | ||||
| AI in combination with dual NRTIs above | ||||
| Raltegravir (Isentress) | 1 tablet (400 mg) twice daily | — | Elevated AST/ALT | Metabolized by UGT1A1; dose increase required with rifampin |
| Elevated creatine phosphokinase | AI† in combination with emtricitabine/tenofovir | |||
| Additional second-choice options‡ | ||||
| NNRTIs | ||||
| Rilpivirine (Edurant) | 1 tablet (25 mg) daily | Weak induction | Depressed mood, insomnia (<10%) | Contraindicated in combination with strong CYP3A4 inducers or proton pump inhibitors |
| AI† in combination with emtricitabine/tenofovir (once-a-day combination; Complera) in patients with HIV viral load <100 000 copies per mL and CD4+ count >200 cells per mm3 | ||||
| Efavirenz (Sustiva; also included in once-a-day combination with emtricitabine/tenofovir (Atripla) | 1 tablet (600 mg) daily; in Atripla, 1 tablet (600/300/ 200 mg) daily | Strong induction | Depressed mood (5%) and increased rate of suicidality | CYP3A4 metabolized; dose adjust if used in combination with voriconazole or rifampin |
| Nervous system symptoms (headache, insomnia, dizziness) <30%, general resolve within 2-4 wk | AI† as Atripla, or in combination with abacavir/lamivudine in patients with HIV viral load <100 000 copies per mL | |||
| Rash (10%-15%) | ||||
| Contraindicated§ | ||||
| PIs | ||||
| Darunavir (Prezista) boosted by ritonavir (Norvir) | 1 tablet (800 mg) daily combined with ritonavir 1 tablet (100 mg) daily | Strong inhibition | Gastrointestinal symptoms (10%-20%) | AI† in combination with emtricitibine/tenofovir |
| Dyslipidemia (20%-25%) | Increased dose recommended for cART-experienced patients or those with specific HIV mutations | |||
| AST/ALT abnormalities (12%) | Contraindicated with strong CYP3A4 inducers | |||
| Rash (6%) | ||||
| Atazanavir (Reyataz) boosted by ritonavir (Norvir) | 1 tablet (300 mg) daily combined with ritonavir 1 tablet (100 mg) daily | Strong inhibition | Hyperbilirubinemia (44%) | A1† in combination with NRTIs above |
| Gastrointestinal symptoms (<5%) | Take with food | |||
| Dyslipidemia (24%) | Concurrent strong CYP3A4 inducers contraindicated | |||
| Rash (5%-7%) | Atazanavir is strong UGT1A1 inhibitor, contraindicated with irinotecan | |||
| Cobicistat-boosted once-a-day regimens | ||||
| Elvitegravir/cobicistat/emtricitabine/tenofovir (Stribild) | 1 tablet (150 mg/150 mg/200 mg/300 mg) daily | Strong inhibition | Gastrointestinal symptoms (10%-20%) | Take with food |
| Headache (7%) | Do not initiate if creatinine clearance <70 mL/min | |||
| Nephrotoxicity (10%) | Concurrent strong CYP3A4 inducers contraindicated | |||
| Class/drugs . | Dose . | CYP3A4 interactions . | Side effects . | Other considerations . |
|---|---|---|---|---|
| First-choice agents* | ||||
| NRTIs | ||||
| Emtricitabine/tenofovir (Truvada) | 1 tablet (200 mg/300 mg) daily | — | Nephrotoxicity (<3%) | Dose adjust for creatinine clearance <50 mL/min |
| Dual anti-HBV activity, preferred in HBV coinfected patients | ||||
| AI† NRTI in several NNRTI-, PI-, and INSTI-based regimens | ||||
| Abacavir/lamivudine (Epzicom) | 1 tablet (600 mg/300 mg) daily | — | Life-threatening hypersensitivity reactions in patients with HLA-B57*01 allele | Pharmacogenomic testing for HLA-B57*01 required before use of abacavir |
| Avoid Epzicom if creatinine clearance <50 mL/min; instead, use renally dosed individual agents | ||||
| AI† NRTI in combination with dolutegravir | ||||
| INSTIs | ||||
| Dolutegravir (Tivicay) | 1 tablet (50 mg) daily | — | Elevated AST/ALT | Metabolized by UGT1A1; dose increase required with efavirenz, rifampin, or select ritonavir-based combinations, and possibly other UGT1A1 inducers |
| Increase dose to 50 mg twice daily for patients with certain INSTI-related mutations | ||||
| AI in combination with dual NRTIs above | ||||
| Raltegravir (Isentress) | 1 tablet (400 mg) twice daily | — | Elevated AST/ALT | Metabolized by UGT1A1; dose increase required with rifampin |
| Elevated creatine phosphokinase | AI† in combination with emtricitabine/tenofovir | |||
| Additional second-choice options‡ | ||||
| NNRTIs | ||||
| Rilpivirine (Edurant) | 1 tablet (25 mg) daily | Weak induction | Depressed mood, insomnia (<10%) | Contraindicated in combination with strong CYP3A4 inducers or proton pump inhibitors |
| AI† in combination with emtricitabine/tenofovir (once-a-day combination; Complera) in patients with HIV viral load <100 000 copies per mL and CD4+ count >200 cells per mm3 | ||||
| Efavirenz (Sustiva; also included in once-a-day combination with emtricitabine/tenofovir (Atripla) | 1 tablet (600 mg) daily; in Atripla, 1 tablet (600/300/ 200 mg) daily | Strong induction | Depressed mood (5%) and increased rate of suicidality | CYP3A4 metabolized; dose adjust if used in combination with voriconazole or rifampin |
| Nervous system symptoms (headache, insomnia, dizziness) <30%, general resolve within 2-4 wk | AI† as Atripla, or in combination with abacavir/lamivudine in patients with HIV viral load <100 000 copies per mL | |||
| Rash (10%-15%) | ||||
| Contraindicated§ | ||||
| PIs | ||||
| Darunavir (Prezista) boosted by ritonavir (Norvir) | 1 tablet (800 mg) daily combined with ritonavir 1 tablet (100 mg) daily | Strong inhibition | Gastrointestinal symptoms (10%-20%) | AI† in combination with emtricitibine/tenofovir |
| Dyslipidemia (20%-25%) | Increased dose recommended for cART-experienced patients or those with specific HIV mutations | |||
| AST/ALT abnormalities (12%) | Contraindicated with strong CYP3A4 inducers | |||
| Rash (6%) | ||||
| Atazanavir (Reyataz) boosted by ritonavir (Norvir) | 1 tablet (300 mg) daily combined with ritonavir 1 tablet (100 mg) daily | Strong inhibition | Hyperbilirubinemia (44%) | A1† in combination with NRTIs above |
| Gastrointestinal symptoms (<5%) | Take with food | |||
| Dyslipidemia (24%) | Concurrent strong CYP3A4 inducers contraindicated | |||
| Rash (5%-7%) | Atazanavir is strong UGT1A1 inhibitor, contraindicated with irinotecan | |||
| Cobicistat-boosted once-a-day regimens | ||||
| Elvitegravir/cobicistat/emtricitabine/tenofovir (Stribild) | 1 tablet (150 mg/150 mg/200 mg/300 mg) daily | Strong inhibition | Gastrointestinal symptoms (10%-20%) | Take with food |
| Headache (7%) | Do not initiate if creatinine clearance <70 mL/min | |||
| Nephrotoxicity (10%) | Concurrent strong CYP3A4 inducers contraindicated | |||
Appropriate HIV therapy generally consists of an INSTI, NNRTI, or PI combined with 2 NTRIs, often in the form of a combination tablet.
Potential drug-drug interaction considerations are underlined.
First choice antiretroviral agents in combination with chemotherapy regimens containing CYP3A4 substrates are those that are not metabolized via CYP3A4 system.
—; no significant effect on CYP3A4 metabolism of other drugs.
Strong level of evidence (AI) in antiretroviral-naïve patients on the basis of randomized controlled trials; recommendations are in concordance with the Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents.56
Use of these second-choice options is somewhat more likely to be limited by potential drug-drug interactions during chemotherapy.
Contraindicated in combination with vinblastine and other chemotherapy drugs heavily dependent on CYP3A4 metabolism; they may be useful after completion of chemotherapy.
Anti-HBV, antibody to HBV; AST/ALT, aspartate aminotransferase/alanine aminotransferase; HLA, human leukocyte antigen; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; UGT1A1, uridine diphosphate glucuronosyltransferase 1 polypeptide A1.
Treatment of relapsed and refractory HIV-cHL
The standard of care for relapsed cHL in HIV-uninfected patients is salvage therapy followed by high-dose chemotherapy and auto-SCT. HIV-infected patients have been shown to successfully undergo this modality.76-79 Treatment-related mortality is 3% to 5% across several conditioning regimens, and long-term outcomes in reported series appear comparable to HIV-negative patients.76-80 It is unclear whether late toxic events, such as secondary myelodysplastic syndrome, are different than in the background population.79
We continue to individualize relapsed therapy according to the treatment history. The case presented here illustrates this philosophy. Currently, 2 clinical trials being conducted by the Blood and Marrow Clinical Trials Network (cosponsored by the NCI and the National Heart, Lung, and Blood Institute) for HIV-infected patients with hematologic cancers should be strongly considered for a patient such as the one highlighted here. One trial is evaluating auto-SCT; the other, allotransplant using preferentially (but not required) homologous CCR5-Δ32 HIV coreceptor donors, possibly rendering the new immune system impervious to HIV. These studies will provide feasibility data and insights into transplant options for HIV-cHL and other hematologic malignancies. Insights may also be gained into HIV reservoirs important toward efforts in HIV eradication. Given the near-universal EBV association, we consider performing allogeneic donor searches early for patients with relapsed or refractory HIV-cHL to expedite potential enrollment in allotransplant studies.
Future directions
Ongoing efforts to decrease toxicity and improve survival in non–HIV-cHL and HIV-cHL are likely to lead to therapeutic advances for both populations. We strongly recommend inclusion of HIV-cHL patients in clinical trials of novel agents and allogeneic transplant to promote advances in the field.81 In HIV-cHL, it is not yet possible to identify those for whom ABVD is likely to fail. Improved prognostic and predictive markers are needed. Response-adapted therapy on the basis of early 18FDG-PET findings has been the subject of recent study in non–HIV-cHL. Negative and positive interim 18FDG-PET scans after 2 cycles of ABVD in advanced-stage non–HIV-cHL have been associated with 95% and 12% 2-year progression-free survival rates, respectively.82-84 The 18FDG-PET results can be falsely positive in HIV, sometimes necessitating confirmation of positive findings. Cycle-1 negative PET scan results may be even more useful in identifying those for whom more limited therapy can be given.85 Although FDG-PET imaging in HIV-cHL has not been validated, preliminary data suggest a high negative predictive value.86 Potentially increased false-positive rates in HIV-cHL may lead to less useful predictive values for positive interim scans. Therefore, strategies using negative 18FDG-PET results for treatment reduction may be the more fruitful research undertaking in HIV-cHL.
Novel therapeutics in primary and second-line cHL therapy is an area of active investigation. Brentuximab vedotin, a CD30-directed immunoconjugate of the antimitotic agent monomethyl auristatin E was approved by the US Food and Drug Administration for refractory cHL.87 Phase 3 studies evaluating the combination of brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine88 as upfront therapy are underway in non–HIV-cHL. The AIDS Malignancy Consortium in collaboration with the NCI Cancer Therapy Evaluation Program is also testing this combination in HIV-cHL (NCT01771107). It is hopeful that outcomes of these studies will advance therapy regardless of HIV serostatus. Many additional immune modulatory (ie, checkpoint inhibition with anti–programmed-death 1 agents) and epigenetic approaches are currently being evaluated in cHL. Advances in these areas and improved understanding of disease biology will likely lead to future paradigm shifts in both non–HIV-cHL and HIV-cHL.
Acknowledgments
We thank Karen Aleman, Kathleen Wyvill, and the clinical staff at the National Institutes of Health Clinical Center for providing patient care; Stefania Pittaluga for histopathology consultation and images; Corina Millo and Millie Whatley for consultation and nuclear medicine images; and Alice Pau for consultation on antiretroviral pharmacology and guidelines.
This work was supported by the Intramural Research Program of the National Institutes of Health and the Cancer Therapy Evaluation Program of the National Cancer Institute.
Authorship
Contribution: T.S.U. and R.F.L. wrote the manuscript, provided patient care, reviewed the case presentation, and performed the literature review; T.S.U. compiled the clinical data; and R.F.L. reviewed the clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard F. Little, 9609 Medical Center Dr, MSC 9739, Bethesda, MD 20892-9739; e-mail: richard_little@nih.gov.