Key Points
Platelet HYAL2 is stored in α-granules and upon activation it becomes surface expressed where it functions to degrade extracellular matrix.
Platelets from IBD patients contain lower HYAL2 protein and activity than those from non-IBD controls.
Abstract
Following injury, platelets rapidly interact with the exposed extracellular matrix (ECM) of the vessel wall and the surrounding tissues. Hyaluronan (HA) is a major glycosaminoglycan component of the ECM and plays a significant role in regulating inflammation. We have recently reported that human platelets degrade HA from the surfaces of activated endothelial cells into fragments capable of inducing immune responses by monocytes. We also showed that human platelets contain the enzyme hyaluronidase-2 (HYAL2), one of two major hyaluronidases that digest HA in somatic tissues. The deposition of HA increases in inflamed tissues in several inflammatory diseases, including inflammatory bowel disease (IBD). We therefore wanted to define the mechanism by which platelets degrade HA in the inflamed tissues. In this study, we show that human platelets degrade the proinflammatory matrix HA through the activity of HYAL2 and that platelet activation causes the immediate translocation of HYAL2 from a distinct population of α-granules to platelet surfaces where it exerts its catalytic activity. Finally, we show that patients with IBD have lower platelet HYAL2 levels and activity than healthy controls.
Introduction
Hyaluronan (HA) is a ubiquitous glycosaminoglycan and a major component of the extracellular matrix (ECM), and it has a crucial role in regulating inflammation.1 HA is produced by the HA synthase enzymes (HAS1-3) and is composed of repeating disaccharides of d-glucuronic acid and N-acetylglucosamine. Not only can HA be synthesized and released, but it can also form a voluminous pericellular coat that surrounds cells. The HA coat is either anchored to the cell surface through binding to specific cell-surface receptors, such as CD44, or it can be retained at the cell surface by sustained transmembrane interactions with its synthases.2 Interestingly, a growing body of literature suggests that different sizes of HA exert a wide spectrum of functions.3 Under normal conditions in tissues, HA is present in its high molecular weight (HMWHA) form (1 to 10 × 106 Da). HMWHA functions as a structural hydrating polymer and is also known to be antiinflammatory,4 protecting from T-cell–mediated liver injury and bleomycin-mediated lung injury in mice.5,6 HMWHA also promotes the suppressive effects of regulatory CD4+CD25+ T cells.7
Increased HA deposition has also been reported in many inflammatory diseases including inflammatory bowel disease (IBD), arthritis, and asthma.8-10 Importantly, degradation of HA results in HA fragments that function as damage-associated molecular patterns.11 Fragmented HA contributes to wound healing, angiogenesis, and inflammation and is capable of signaling cellular responses through specific receptors.12 For example, HA fragments can activate macrophages and dendritic cells and can stimulate the transcription of inflammatory genes including tumor necrosis factor α, interleukin-12 (IL-12), and IL-1b.4 In monocytes, HA fragments were able to stimulate the production of IL-6 and monocyte chemoattractant protein-1 through CD44 and toll-like receptor 4 (TLR4).13 Recent studies have shown that HA fragments can also induce innate host defense responses at the intestinal epithelium by promoting the expression of human β-defensin-2, a potent antimicrobial peptide.14
The accumulation of HA fragments in tissues during injury or under inflammation is thought to be largely the result of enzymatic degradation of HA, mainly through hyaluronidase-1 (HYAL1) and HYAL2 in somatic tissues.12,15 HYAL2 is a glycophosphatidylinositol-anchored protein that digests HA with the cooperation of CD44, the classical HA receptor.16-18 Digested HA is then either internalized into the lysosome for further digestion or released into the environment.17,19 KIAA1199 has recently been identified as an HA-binding protein and a contributor to HA degradation.20 HA digestion can also occur by nonenzymatic mechanisms, mainly through reactive oxygen species.21,22
We previously reported that mouse and human platelets and their megakaryocytic precursors express both HYAL2 messenger RNA and protein with no evidence for HYAL1. We also showed that platelets can degrade endothelial proinflammatory matrix HA into signaling sized HA fragments that stimulate monocytes.19 Platelets can act as inflammatory cells beyond their main role in thrombus formation.23 They store numerous inflammatory mediators in their granules, which can be released upon activation.24 For example, CD154 on the surface of activated platelets can promote leukocyte recruitment to sites of inflammation.25 Platelets also express a number of the TLR family members. Platelet TLR4, for instance, can bind bacterial lipopolysaccharide, whereas TLR9 expressed on activated platelets can mediate bacterial DNA sequestration.26,27
In this article, we define a novel mechanism by which platelets modify the ECM. We demonstrate that after activation, platelets degrade the proinflammatory matrix by using HYAL2. This activation-dependent mechanism results in the immediate translocation of HYAL2 from α-granules to platelet surfaces where it exerts its catalytic activity. Furthermore, we show that platelets of patients with IBD have lower HYAL2 protein content and hyaluronidase activity than control platelets, which might explain the accumulation of the leukocyte-adhesive HA observed in IBD tissues.10
Methods
Cell coculture assay for platelet hyaluronidase activity and fragment generation
Confluent human intestinal mucosal smooth muscle cells (M-SMCs) isolated from colon surgical specimens (Department of Surgical Pathology at the Cleveland Clinic) were grown in 24-well cell culture plates and treated without or with 50 µg/mL polyinosinic:polycytidylic acid (polyI:C) for 18 hours. Culture media was replaced with RPMI media containing 1% fetal bovine serum alone or containing freshly isolated resting platelets or thrombin receptor-activating peptide 6 (TRAP-6) –activated platelets (25 µM for 1 minute at room temperature). Cocultures were incubated for 2 hours at 37°C. In some experiments, platelets were pre-incubated with blocking antibody against HYAL2 (1, 12, and 25 µg/mL) (Thermo) or control immunoglobulin (IgG; 25 µg/mL). Culture media were collected and centrifuged to remove platelets. Total HA released into the media was measured by using a competitive enzyme-linked immunosorbent assay (ELISA) –like assay (Echelon) according to the manufacturer’s protocol. Unbound platelets, obtained by collection and centrifugation of culture media, and SMC-bound platelets, obtained by scraping SMCs, were analyzed by flow cytometry for their surface P-selectin and HYAL2 expression.
HA isolation and sizing
HA was purified from stimulated M-SMCs before and after they were co-incubated with platelets. M-SMCs were incubated with 0.5 mg/mL proteinase-K in phosphate-buffered saline for 18 hours at 60°C and then with benzonase (50 U/mL) for 1 hour at 37°C. Samples were then concentrated by using speedvac and dialyzed against 0.1 M NaCl. Samples were applied to anion exchange spin columns (Thermo) and centrifuged for 5 minutes. Columns were washed with 0.1 M and 0.4 M NaCl before HA was eluted with 0.8 M NaCl. Eluted samples containing HA were dialyzed against water, concentrated, and analyzed by using 0.5% agarose sizing gel as described previously.28 Stained bands in the gel were confirmed to be HA by demonstrating sensitivity to Streptomyces hyaluronidase (HAase; 2 U/mL for 1 hour at 37°C).
Hyaluronidase assay using immobilized purified HA
Freshly isolated resting, TRAP-activated, or lysed platelets resuspended in neutral or acidic RPMI medium were added to a 96-well plate coated with HA (Echelon) (3 × 108 platelets per well). The plate was incubated at 37°C for 18 hours and then washed. Residual HA was detected colorimetrically by using an HA detection system provided by the manufacturer. HAase (2 U/mL) was used as a positive control and for defining maximum activity.
Hyaluronidase assay using purified HA in solution
Platelets were incubated at 37°C for 18 hours in RPMI medium containing 1000 kDa Select-HA (5 µg/mL), an enzymatically synthesized commercial HA with high size control. Samples from the incubation were analyzed for HA by 0.5% agarose gel electrophoresis as described previously28 (0.5 µg of HA per lane).
Subcellular fractionation of platelets using sucrose density gradient
Subcellular fractionation of platelets using sucrose density gradient was achieved as previously described.29 Briefly, freshly isolated platelets were sonicated and applied on top of a linear 30% to 60% sucrose gradient followed by ultracentrifugation at 100 000g for 90 minutes. Nine fractions were collected by pipetting from the top, and equal volumes of fractions were analyzed by immunoblotting for the presence of HYAL2, P-selectin, von Willbrand factor (vWF), CD42b, and LAMP-1.
Microscopy and flow cytometry
Detailed protocols and lists of antibodies and concentrations for immunofluorescence microscopy, immunoelectron microscopy, and flow cytometry are provided in the supplemental Data (available online at the Blood Web site).
Results
Platelets degrade proinflammatory matrix HA
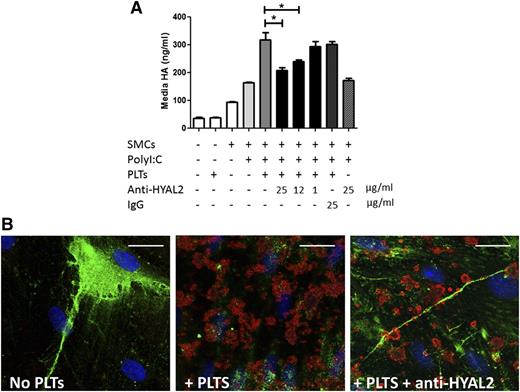
We previously demonstrated that platelets can digest HA from surfaces of activated endothelium, which produces fragmented HA capable of promoting cellular responses.19 To investigate the mechanism by which platelets cleave matrix HA, we adapted a cell coculture assay. Cultured human intestinal M-SMCs isolated from surgical specimens were treated with polyI:C, a double-stranded RNA used to mimic a virus infection, to stimulate production of proinflammatory leukocyte-adhesive HA.10,30 Freshly isolated human platelets were coincubated with polyI:C-stimulated M-SMCs for 2 hours at 37°C. Cell-associated HA was then purified and analyzed by agarose gel electrophoresis. The highly polydisperse cell–associated HA in the size range of 0.1 to 2 ×106 Da was detected on the stimulated cells (Figure 1A, lane 1). However, HA was not detected on cells co-incubated with platelets (Figure 1A, lane 2) indicating that platelets cleared the proinflammatory matrix HA on the M-SMC surface. Replicate samples were subjected to HAase, an enzyme that specifically degrades HA, to confirm that the stained bands were HA (Figure 1A, lanes 3 and 4). To test whether the HA cleaved by platelets is released into the culture medium, we measured HA in the culture fluid by using an ELISA-like assay. As expected, polyI:C-treated cells released significantly higher amounts of HA than nontreated cells (P < .001). However, when polyI:C-treated cells were incubated with platelets, the amount of HA released was significantly increased (P < .001) (Figure 1B). The effect that platelets have on HA in the extracellular matrix is striking when observed by histochemical staining. Upon polyI:C treatment, M-SMCs produced large amounts of leukocyte-adhesisve HA structures or HA cables (Figure 1C, green). Following co-incubation with platelets, HA cables were completely removed from the surfaces of M-SMCs, confirming the role of platelets in clearing proinflammatory HA. To assess the activation state of platelets in culture and whether culture conditions (eg, serum and SMC surface molecules) contribute to platelet activation, we analyzed their surface P-selectin by using flow cytometry. We found that platelets that were bound to SMCs demonstrated significantly higher surface expression of P-selectin compared with unbound platelets. Importantly, bound platelets also showed a significant increase in surface HYAL2 compared with unbound platelets (Figure 1D).
Platelets digest HA in the inflammatory matrix. PolyI:C-stimulated human M-SMCs were co-incubated with freshly isolated human platelets (PLTs) for 2 hours at 37°C. (A) Agarose gel electrophoretic analysis of HA purified from polyI:C-stimulated M-SMCs before (lane 1) and after (lane 2) coincubation with platelets. Polydisperse HA with a size range of 0.1 to 2 × 106 Da associated with M-SMCs was no longer detected after co-incubation with platelets. Replicate samples were treated with HAase, an enzyme that specifically digests HA, to confirm that the stained bands were HA (lanes 3 and 4). HA ladder units: 106 Da. (B) ELISA-like assay measurement of HA released into the media by M-SMCs. Coincubation of M-SMCs with platelets results in a significant increase of HA in culture media. Data represent mean ± standard error (SE) of 10 separate experiments. ***P < .001; N.S., not significant. (C) Histochemical staining of M-SMC-associated HA (green). M-SMCs were fixed in cold methanol, and HA was detected with biotinylated HA-binding protein and Alexa Fluor 488–conjugated streptavidin. M-SMCs responded to polyI:C treatment by producing high amounts of HA. The co-incubation of platelets with polyI:C-stimulated M-SMCs caused the removal of the M-SMC surface–associated HA. Image and capture details: Leica upright microscope DM5500 B (Leica, Wetzlar, Germany), HC PLAN APO ×20/0.7NA dry objective, QImaging Retiga cooled CCD camera, QCapture Suite Software (QImaging, Surrey, BC Canada). Scale bar: 100 µm. (D) Fold-increase in surface P-selectin and HYAL2 mean fluorescence intensity (MFI) of SMC-bound platelets in comparison with unbound platelets following culture as measured by flow cytometry. SMC-bound platelets demonstrated significantly higher surface P-selectin and HYAL2 compared with unbound platelets after culture. Platelets were defined by forward (FSC-A), and side scatter (SSC-A) characteristics.
Platelets digest HA in the inflammatory matrix. PolyI:C-stimulated human M-SMCs were co-incubated with freshly isolated human platelets (PLTs) for 2 hours at 37°C. (A) Agarose gel electrophoretic analysis of HA purified from polyI:C-stimulated M-SMCs before (lane 1) and after (lane 2) coincubation with platelets. Polydisperse HA with a size range of 0.1 to 2 × 106 Da associated with M-SMCs was no longer detected after co-incubation with platelets. Replicate samples were treated with HAase, an enzyme that specifically digests HA, to confirm that the stained bands were HA (lanes 3 and 4). HA ladder units: 106 Da. (B) ELISA-like assay measurement of HA released into the media by M-SMCs. Coincubation of M-SMCs with platelets results in a significant increase of HA in culture media. Data represent mean ± standard error (SE) of 10 separate experiments. ***P < .001; N.S., not significant. (C) Histochemical staining of M-SMC-associated HA (green). M-SMCs were fixed in cold methanol, and HA was detected with biotinylated HA-binding protein and Alexa Fluor 488–conjugated streptavidin. M-SMCs responded to polyI:C treatment by producing high amounts of HA. The co-incubation of platelets with polyI:C-stimulated M-SMCs caused the removal of the M-SMC surface–associated HA. Image and capture details: Leica upright microscope DM5500 B (Leica, Wetzlar, Germany), HC PLAN APO ×20/0.7NA dry objective, QImaging Retiga cooled CCD camera, QCapture Suite Software (QImaging, Surrey, BC Canada). Scale bar: 100 µm. (D) Fold-increase in surface P-selectin and HYAL2 mean fluorescence intensity (MFI) of SMC-bound platelets in comparison with unbound platelets following culture as measured by flow cytometry. SMC-bound platelets demonstrated significantly higher surface P-selectin and HYAL2 compared with unbound platelets after culture. Platelets were defined by forward (FSC-A), and side scatter (SSC-A) characteristics.
Degradation of matrix HA by platelets is HYAL2 dependent
The two somatically active HA degrading enzymes in humans are HYAL1 and HYAL2.31 We showed previously that platelets contain only HYAL2 protein and messenger RNA, with no evidence of HYAL1.19 Additionally, we observed in the previous experiments that HYAL2 increases on the surface of platelets bound to SMCs in culture. Therefore, we hypothesized that proinflammatory HA clearance by platelets is mediated by platelet HYAL2. To test this hypothesis, we incubated platelets with different concentrations of HYAL2-neutralizing antibodies or with nonspecific control IgG before they were co-incubated with polyI:C-stimulated M-SMCs. We then analyzed HA released into the media by the ELISA-like assay. HA concentrations in media collected from M-SMCs that were incubated with HYAL2 antibody–treated platelets were significantly lower than in media collected from cells incubated with either untreated platelets or platelets preincubated with IgG (Figure 2A) (n = 3; P < .05). We confirmed that the effect of the blocking antibody is specific to platelet HYAL2, not SMC HYAL2, by centrifuging the HYAL2 antibody–treated platelets to remove excess antibody before they were added to the culture (data not shown). To further confirm HYAL2 antibody blockade, we performed histochemical staining of HA on polyI:C-stimulated M-SMC (green) co-incubated with platelets (red for CD42b) in the absence or presence of HYAL2 antibody (Figure 2B). HA cables on the surfaces of M-SMCs that were co-incubated with HYAL2 antibody–treated platelets remained intact, whereas HA on M-SMCs co-incubated with untreated platelets was removed, consistent with our ELISA-like data. Interestingly, HYAL2 antibody–treated platelets retained their HA cable-binding ability, an observation previously reported when monocytes or platelets were incubated with HA cables at 4°C.10,19
Platelets degrade proinflammatory matrix HA using HYAL2. PolyI:C-stimulated human M-SMCs were co-incubated with untreated freshly isolated human platelets or with HYAL2 blocking antibody–treated platelets for 2 hours at 37°C. (A) ELISA-like assay measurement of the amounts of HA released into the media by M-SMCs. Coincubation of stimulated M-SMCs with HYAL2 antibody–treated platelets resulted in a significant decrease in HA released into media compared with untreated or IgG-treated platelets (n = 3; *P < .05). The inhibiting effect of the HYAL2 blocking antibody was dose dependent. (B) Histochemical staining of M-SMC–associated HA (green) and platelets (red = CD42b) in the absence or presence of HYAL2 antibody. Whereas untreated platelets degraded HA on stimulated M-SMCs, HYAL2 antibody–treated platelets bound HA cables on the surfaces of M-SMCs without degrading them. Image, detection, and software details: Leica TCS SP5 II confocal/multi-photon high-speed upright microscope (Leica), HCX PL APO ×40/1.25NA oil immersion objective, Leica HyD system detector, Leica LAS AF software (Leica). Scale bar: 25 µm.
Platelets degrade proinflammatory matrix HA using HYAL2. PolyI:C-stimulated human M-SMCs were co-incubated with untreated freshly isolated human platelets or with HYAL2 blocking antibody–treated platelets for 2 hours at 37°C. (A) ELISA-like assay measurement of the amounts of HA released into the media by M-SMCs. Coincubation of stimulated M-SMCs with HYAL2 antibody–treated platelets resulted in a significant decrease in HA released into media compared with untreated or IgG-treated platelets (n = 3; *P < .05). The inhibiting effect of the HYAL2 blocking antibody was dose dependent. (B) Histochemical staining of M-SMC–associated HA (green) and platelets (red = CD42b) in the absence or presence of HYAL2 antibody. Whereas untreated platelets degraded HA on stimulated M-SMCs, HYAL2 antibody–treated platelets bound HA cables on the surfaces of M-SMCs without degrading them. Image, detection, and software details: Leica TCS SP5 II confocal/multi-photon high-speed upright microscope (Leica), HCX PL APO ×40/1.25NA oil immersion objective, Leica HyD system detector, Leica LAS AF software (Leica). Scale bar: 25 µm.
Degradation of purified HA by platelets and platelet lysates occurs only at low pH
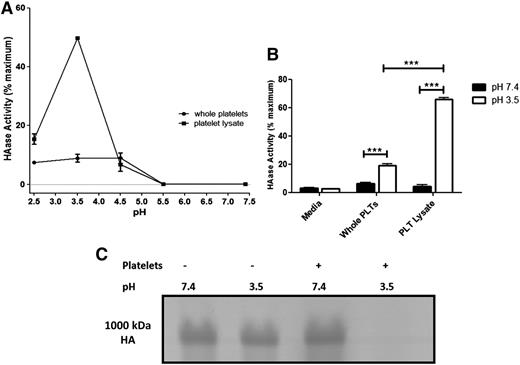
HYAL2 is reported to be an acid active enzyme.32 With the observation that platelets degrade matrix HA by HYAL2 under physiological conditions (pH 7.2), we tested whether platelets have the ability to degrade purified HA under neutral pH using two approaches: Can platelets degrade purified HA immobilized to a surface? and Can platelets degrade purified HA in solution? We first used a range of pH conditions. Platelets that were incubated with immobilized purified HA at neutral pH displayed no HA degrading activity, whereas platelets incubated under acidic conditions (pH 2.5 to 4.5) demonstrated hyaluronidase activity. Platelet lysates also had a peak hyaluronidase activity at pH 3.5, with no measurable activity at neutral pH (Figure 3A). Surprisingly, platelet lysates showed significantly higher hyaluronidase activity (60% maximum) than whole platelets (20% maximum) (n = 3; P < .001) (Figure 3B). Maximum activity was achieved by HAase. The data suggest that the majority of the resting platelet HYAL2 is not on the surface. To test whether platelets can degrade purified HA in solution, platelets were incubated with Select-HA (1000 kDa), a commercial monodispersed HA prepared through enzymatic synthesis in which a high level of size control is possible. As observed with immobilized HA, platelets were able to digest only purified HA in solution under acidic pH (Figure 3C).
Platelets and platelet lysates can only degrade purified HA under acidic pH. (A-B) Platelets and platelet lysates were incubated with purified HA immobilized to wells of a 96-well plate for 18 hours at 37°C (3 × 108 platelets per well). At the end of the incubation, platelets and degraded HA were washed away, and hyaluronidase activity was measured by detecting residual HA colorimetrically. Maximum activity was achieved by HAase. (A) hyaluronidase activity of platelets and their lysates at different pHs. Platelets and their lysates demonstrated purified HA-degrading activity only under acidic pH (optimum pH, 3.5) with no detectable activity at neutral pH. (B) Purified HA-degrading activity of platelets compared with the activity of their lysates. Platelet lysates have significantly higher purified HA–degrading activity than intact platelets. n = 3; ***P < .001. (C) Purified HA (Select-HA, 1000 kDa) in RPMI medium (5 mg/mL) was incubated with platelets for 18 hours at 37°C. Platelets degraded purified HA only under acidic pH (pH 3.5) but not under neutral pH.
Platelets and platelet lysates can only degrade purified HA under acidic pH. (A-B) Platelets and platelet lysates were incubated with purified HA immobilized to wells of a 96-well plate for 18 hours at 37°C (3 × 108 platelets per well). At the end of the incubation, platelets and degraded HA were washed away, and hyaluronidase activity was measured by detecting residual HA colorimetrically. Maximum activity was achieved by HAase. (A) hyaluronidase activity of platelets and their lysates at different pHs. Platelets and their lysates demonstrated purified HA-degrading activity only under acidic pH (optimum pH, 3.5) with no detectable activity at neutral pH. (B) Purified HA-degrading activity of platelets compared with the activity of their lysates. Platelet lysates have significantly higher purified HA–degrading activity than intact platelets. n = 3; ***P < .001. (C) Purified HA (Select-HA, 1000 kDa) in RPMI medium (5 mg/mL) was incubated with platelets for 18 hours at 37°C. Platelets degraded purified HA only under acidic pH (pH 3.5) but not under neutral pH.
HYAL2 becomes surface expressed on thrombin receptor–activated platelets
The observations that HYAL2 increased on the surface of SMC-bound platelets, which was also accompanied by an increase in surface P-selectin expression, and that platelet lysates had significantly higher hyaluronidase activity than intact platelets suggested that platelet activation may have a role in the surface expression of HYAL2. Therefore, we analyzed the normal HYAL2 expression parameters in resting and activated platelets. First, we examined whether activated platelets package HYAL2 into microparticles. Immunoblot analysis revealed that, upon activation, HYAL2 remains associated with platelets and does not associate with microparticles or any platelet releasate, even after 30 minutes of incubation with TRAP (Figure 4A-B). The same was true when we activated platelets with thrombin plus collagen (data not shown). We then compared HYAL2 expression in paraformaldehyde fixed nonpermeabilized platelets by immunohistochemical staining using fluorescence microscopy (Figure 4C). Surface expression of P-selectin (red) was used as a platelet activation marker. TRAP-activated platelets showed significantly higher HYAL2 staining levels than resting platelets. Because platelets were not permeabilized, the data provided evidence that HYAL2 is targeted to platelet surfaces only upon activation. Next we analyzed surface expression of HYAL2 in unstimulated and TRAP-stimulated platelets by flow cytometry. To eliminate the spontaneously activated platelets in the unstimulated population, we gated on the strictly P-selectin–negative population in that sample. The P-selectin–negative population demonstrated no surface HYAL2 expression, whereas the P-selectin–positive population in the TRAP-stimulated sample showed significant surface HYAL2 expression (Figure 4D). By using flow cytometry analysis, we also determined that only 6.8% ± 1.3% (± standard error) of nonactivated (P-selectin negative) platelets in the tested population expressed surface HYAL2 compared with 37.6% ± 6.4% of activated platelets (n = 9; P < .001) (Figure 4D). Flow cytometry analysis also showed a threefold increase in mean fluorescence intensity of surface HYAL2 in TRAP-activated platelets compared with nonactivated platelets (n = 9; P < .001) (Figure 4E).
HYAL2 becomes surface expressed upon thrombin receptor–mediated platelet activation. (A) Freshly isolated human platelets were treated without or with TRAP (15 µM) for 10 minutes before microparticles were isolated. Immunoblot analysis showed that, upon activation, HYAL2 was associated with platelets (750-g pellet) and not with microparticles (104-g pellet) or platelet releasate (104 g supernatant). (B) Immunoblot analysis showing that HYAL2 remains associated with platelets (750-g pellet) and not with microparticles or releasate (REL) even after 30 minutes incubation with TRAP. (C) Freshly isolated human platelets were treated without or with TRAP (25 µM) for 1 minute before they were fixed with 3.5% paraformaldehyde for 30 minutes. Fixed platelets were spun down onto slides coated with poly-l-lysine and stained for P-selectin (red) and HYAL2 (green). Similar to P-selectin, HYAL2 was detected only on the surfaces of activated platelets. Image and software details: Leica upright microscope DM5500 B (Leica), HCX PLAN APO ×63/1.32NA oil immersion objective, QImaging Retiga cooled CCD camera, QCapture Suite Software (QImaging). Scale bar: 20 µm. (D-F) Freshly isolated platelets were treated without or with TRAP (25 µM) for 1 minute at room temperature before they were fixed in 1% paraformaldehyde for 2 hours at room temperature. Fixed platelets were stained for HYAL2 and P-selectin and analyzed by using flow cytometry. (D) Representative histograms of surface P-selectin and HYAL2 staining in unstimulated and TRAP-stimulated platelets. The P-selectin–negative population in unstimulated platelets demonstrated no surface HYAL2 expression, whereas the P-selectin–positive population in the TRAP-stimulated sample showed significant surface HYAL2 expression. PE, phycoerythrin. (E) Percentage of surface HYAL2-positive platelets measured by flow cytometry. As a result, 37.6% ± 6.4% (±SE) of TRAP-activated platelets expressed surface HYAL2 compared with only 6.8% ± 1.3% of nonactivated (P-selectin negative) platelets. n = 9; ***P < .001. (F) Fold-increase in surface HYAL2 MFI of TRAP-activated platelets in comparison with nonactivated (P-selectin negative) platelets. Activated platelets demonstrated a threefold increase in surface HYAL2 expression compared with nonactivated platelets. n = 9; ***P < .001.
HYAL2 becomes surface expressed upon thrombin receptor–mediated platelet activation. (A) Freshly isolated human platelets were treated without or with TRAP (15 µM) for 10 minutes before microparticles were isolated. Immunoblot analysis showed that, upon activation, HYAL2 was associated with platelets (750-g pellet) and not with microparticles (104-g pellet) or platelet releasate (104 g supernatant). (B) Immunoblot analysis showing that HYAL2 remains associated with platelets (750-g pellet) and not with microparticles or releasate (REL) even after 30 minutes incubation with TRAP. (C) Freshly isolated human platelets were treated without or with TRAP (25 µM) for 1 minute before they were fixed with 3.5% paraformaldehyde for 30 minutes. Fixed platelets were spun down onto slides coated with poly-l-lysine and stained for P-selectin (red) and HYAL2 (green). Similar to P-selectin, HYAL2 was detected only on the surfaces of activated platelets. Image and software details: Leica upright microscope DM5500 B (Leica), HCX PLAN APO ×63/1.32NA oil immersion objective, QImaging Retiga cooled CCD camera, QCapture Suite Software (QImaging). Scale bar: 20 µm. (D-F) Freshly isolated platelets were treated without or with TRAP (25 µM) for 1 minute at room temperature before they were fixed in 1% paraformaldehyde for 2 hours at room temperature. Fixed platelets were stained for HYAL2 and P-selectin and analyzed by using flow cytometry. (D) Representative histograms of surface P-selectin and HYAL2 staining in unstimulated and TRAP-stimulated platelets. The P-selectin–negative population in unstimulated platelets demonstrated no surface HYAL2 expression, whereas the P-selectin–positive population in the TRAP-stimulated sample showed significant surface HYAL2 expression. PE, phycoerythrin. (E) Percentage of surface HYAL2-positive platelets measured by flow cytometry. As a result, 37.6% ± 6.4% (±SE) of TRAP-activated platelets expressed surface HYAL2 compared with only 6.8% ± 1.3% of nonactivated (P-selectin negative) platelets. n = 9; ***P < .001. (F) Fold-increase in surface HYAL2 MFI of TRAP-activated platelets in comparison with nonactivated (P-selectin negative) platelets. Activated platelets demonstrated a threefold increase in surface HYAL2 expression compared with nonactivated platelets. n = 9; ***P < .001.
Platelet HYAL2 is localized to a distinct population of α-granules
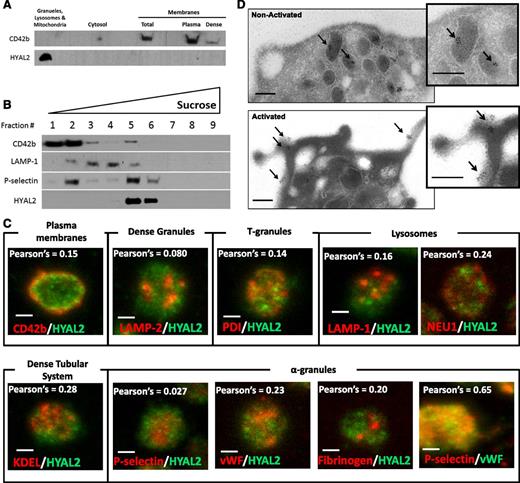
Because HYAL2 seems to be expressed on the platelet surface only upon activation, we hypothesized that HYAL2 is stored inside platelet granules in resting platelets. To test this, we wanted to determine whether HYAL2 partitions with granules, cytosol, or membranes (plasma membranes and dense tubular system) in resting platelets. First, we separated platelet granules (α-granules, dense granules, lysosomes, and mitochondria) from platelet membranes by differential centrifugation.29,30 The platelet membrane marker CD42b was detected, as expected, in the plasma membrane fraction (Figure 5A). HYAL2, however, was detected specifically in the granule fraction. Next, to define the granule type in which HYAL2 is stored, sucrose density gradients were used to separate platelet subcellular compartments (Figure 5B). HYAL2 and P-selectin, an α-granule marker, were enriched in the same sucrose fraction, suggesting that HYAL2 is stored in platelet α-granules. CD42b and LAMP-1 were used as plasma membrane and lysosomal markers, respectively. Subsequently, we analyzed colocalization of platelet HYAL2 with different platelet granule markers by using fluorescence microscopy (Figure 5C). Consistent with the sucrose density gradient data, platelet HYAL2 staining did not colocalize with known specific markers of plasma membranes (CD42b), lysosomes (LAMP-1 and NEU1), dense granules (LAMP-2), T-granules (protein disulfide isomerase), or the dense tubular system (KDEL). Surprisingly, platelet HYAL2 also did not colocalize with the known specific markers of α-granules (vWF, P-selectin, and fibrinogen). The lack of colocalization between platelet HYAL2 and the α-granule markers we examined led us to conclude that HYAL2 is stored in a different subset of α-granules inside resting platelets. As a positive control, we analyzed the colocalization of vWF and P-selectin, 2 known α-granule markers, in platelets. As expected, vWF and P-selectin showed significant levels of colocalization (Pearson correlation coefficient, 0.65). To finally determine that HYAL2 is stored in platelet α-granules, given the fact that it did not colocalize with known α-granule markers, we examined its localization by immunoelectron microscopy. Inside resting platelets, HYAL2 was clearly detected within α-granules, which were identified by their distinct morphology and number. However, whereas resting platelet surfaces seemed to be devoid of any HYAL2 expression, HYAL2 was clearly detected on the surfaces of TRAP-activated platelets (Figure 5D). Interestingly, HYAL2 seems to exist in clusters on platelet surfaces, unlike the previously reported uniform distribution of adhesion molecules such as P-selectin.33,34
HYAL2 is stored in a distinct subset of α-granules in resting platelets. (A) Immmunoblot analysis for the presence of CD42b and HYAL2 of different platelet subcellular fractions obtained by differential centrifugation. HYAL2 was detected exclusively in the fraction containing granules, lysosomes, and mitochondria (19 000-g pellet), whereas CD42b was detected in the membranes fraction (100 000-g pellet). (B) Platelets were sonicated and applied on top of a linear sucrose density gradient before the gradient was centrifuged at 100 000g for 90 minutes. Nine fractions were collected from the top of the gradient. Equal volumes of fractions were analyzed by immunoblotting for the presence of CD42b (plasma membrane marker), LAMP-1 (lysosomal marker), P-selectin (α-granule marker), and HYAL2. HYAL2 and P-selectin were both enriched in fraction 5, the α-granules fraction, whereas CD42b and LAMP-1 were enriched in fractions 2 and 4, respectively. (C) Confocal microscopy images (maximum projection) of platelets stained for HYAL2 (green) and one of the following proteins: CD42b (plasma membrane marker), LAMP-2 (dense granule marker), protein disulfide isomerase (T-granule marker), KDEL (dense tubular system marker), LAMP-1 and NEU1 (lysosomal markers), P-selectin (α-granule marker), vWF (α-granule marker), and fibrinogen (α-granule marker). HYAL2 did not colocalize with any of the tested markers. Pearson’s correlation coefficients were obtained by analyzing individual images (layers) of the Z-stack using Image-Pro Plus software (Media Cybernetics, Rockville, MD). Image, detection, and software details: Leica TCS SP5 II confocal/multiphoton high-speed upright microscope (Leica), HCX PL APO 63Χ/1.4NA oil immersion objective, Leica HyD system detector, Leica LAS AF software (Leica). Scale bar: 1 µm. (D) Immunoelectron microscopy images showing resting and activated platelets. Washed human platelets were fixed, sectioned, and mounted on formvar-coated nickel grids. Ultrathin platelet sections were probed for HYAL2, and the bound antibody was labeled with immunogold (10 nm). In resting platelets, HYAL2 appeared to be localized within platelet α-granules (black arrows), whereas platelet surface appeared to be devoid of any HYAL2. However, in activated platelets, HYAL2 was clearly detected on the surface. Image and detection details: FEI Tecnai G2 Spirit BioTWIN Transmission Electron Microscope (FEI Company, Hillsboro, OR), Orius 832 CCD 11-megapixel camera, Digitalmicrograph (Gatan, Inc., Pleasanton, CA). Scale bars: 200 nm.
HYAL2 is stored in a distinct subset of α-granules in resting platelets. (A) Immmunoblot analysis for the presence of CD42b and HYAL2 of different platelet subcellular fractions obtained by differential centrifugation. HYAL2 was detected exclusively in the fraction containing granules, lysosomes, and mitochondria (19 000-g pellet), whereas CD42b was detected in the membranes fraction (100 000-g pellet). (B) Platelets were sonicated and applied on top of a linear sucrose density gradient before the gradient was centrifuged at 100 000g for 90 minutes. Nine fractions were collected from the top of the gradient. Equal volumes of fractions were analyzed by immunoblotting for the presence of CD42b (plasma membrane marker), LAMP-1 (lysosomal marker), P-selectin (α-granule marker), and HYAL2. HYAL2 and P-selectin were both enriched in fraction 5, the α-granules fraction, whereas CD42b and LAMP-1 were enriched in fractions 2 and 4, respectively. (C) Confocal microscopy images (maximum projection) of platelets stained for HYAL2 (green) and one of the following proteins: CD42b (plasma membrane marker), LAMP-2 (dense granule marker), protein disulfide isomerase (T-granule marker), KDEL (dense tubular system marker), LAMP-1 and NEU1 (lysosomal markers), P-selectin (α-granule marker), vWF (α-granule marker), and fibrinogen (α-granule marker). HYAL2 did not colocalize with any of the tested markers. Pearson’s correlation coefficients were obtained by analyzing individual images (layers) of the Z-stack using Image-Pro Plus software (Media Cybernetics, Rockville, MD). Image, detection, and software details: Leica TCS SP5 II confocal/multiphoton high-speed upright microscope (Leica), HCX PL APO 63Χ/1.4NA oil immersion objective, Leica HyD system detector, Leica LAS AF software (Leica). Scale bar: 1 µm. (D) Immunoelectron microscopy images showing resting and activated platelets. Washed human platelets were fixed, sectioned, and mounted on formvar-coated nickel grids. Ultrathin platelet sections were probed for HYAL2, and the bound antibody was labeled with immunogold (10 nm). In resting platelets, HYAL2 appeared to be localized within platelet α-granules (black arrows), whereas platelet surface appeared to be devoid of any HYAL2. However, in activated platelets, HYAL2 was clearly detected on the surface. Image and detection details: FEI Tecnai G2 Spirit BioTWIN Transmission Electron Microscope (FEI Company, Hillsboro, OR), Orius 832 CCD 11-megapixel camera, Digitalmicrograph (Gatan, Inc., Pleasanton, CA). Scale bars: 200 nm.
Activated platelets have higher hyaluronidase activity than nonactivated platelets
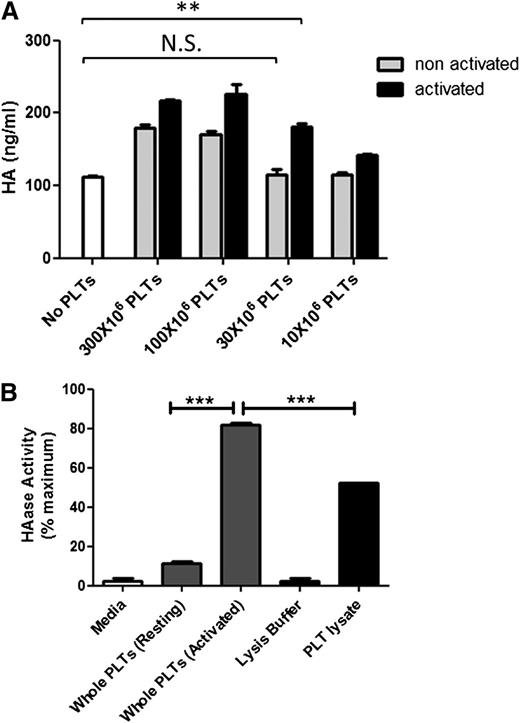
Platelet HYAL2 appeared on the surface of platelets after activation. Therefore, we postulated that platelet hyaluronidase activity also increases upon activation. We first examined whether activated platelets have higher HA degrading ability than nonactivated platelets in the coculture model. Replicate cultures of polyI:C-treated M-SMCs were cocultured with decreasing concentrations (300 to 10 × 106 platelets per well) of either TRAP-activated or nonactivated freshly isolated platelets. The levels of HA released into the media by activated platelets were higher than HA released by nontreated platelets under all conditions, suggesting that activated platelets had higher HA degrading ability than nonactivated platelets (Figure 6A). However, below a platelet concentration of 30 × 106 platelets per well, nontreated platelets had no HA-degrading ability, whereas activated platelets retained significant levels of activity. We then examined whether activated platelets also have higher purified HA–degrading activity compared with nonactivated platelets. TRAP-activated and nonactivated platelets were incubated with purified HA immobilized to wells of a 96-well plate (pH 3.5). Activated platelets demonstrated significantly higher hyaluronidase activity (80% maximum) than nonactivated platelets (10% maximum; P < .001). Interestingly, activated platelets also showed significantly higher hyaluronidase activity than platelet lysates (Figure 6B). As expected, activated platelets also did not display purified HA–degrading ability under neutral pH (data not shown).
Thrombin receptor-mediated platelet activation results in increased platelet hyaluronidase activity. (A) PolyI:C-stimulated human M-SMCs were coincubated with decreasing concentrations of either untreated freshly isolated platelets or TRAP-activated platelets for 2 hours at 37°C. The amount of HA released into the media was measured by ELISA-like assay. Activated platelets caused a significantly higher increase in HA released into the media than nonactivated platelets. At a concentration of 30 × 106 platelets per well, nonactivated platelets appeared to have no hyaluronidase activity compared with activated platelets, which still released significant amounts of HA into media. n = 3; **P < .01. (B) Freshly isolated untreated or TRAP-activated or lysed platelets (3 × 108 platelets per well) were incubated with purified HA immobilized to wells of a 96-well plate for 18 hours at 37°C (pH 3.5). Platelets and digested HA were then washed, and the HA remaining on the wells was detected colorimetrically. Activated platelets demonstrated significantly higher hyaluronidase activity (80% maximum) than nonactivated platelets (10% maximum) and platelet lysate (50% maximum). n = 3; ***P < .001. Maximum activity was achieved by HAase.
Thrombin receptor-mediated platelet activation results in increased platelet hyaluronidase activity. (A) PolyI:C-stimulated human M-SMCs were coincubated with decreasing concentrations of either untreated freshly isolated platelets or TRAP-activated platelets for 2 hours at 37°C. The amount of HA released into the media was measured by ELISA-like assay. Activated platelets caused a significantly higher increase in HA released into the media than nonactivated platelets. At a concentration of 30 × 106 platelets per well, nonactivated platelets appeared to have no hyaluronidase activity compared with activated platelets, which still released significant amounts of HA into media. n = 3; **P < .01. (B) Freshly isolated untreated or TRAP-activated or lysed platelets (3 × 108 platelets per well) were incubated with purified HA immobilized to wells of a 96-well plate for 18 hours at 37°C (pH 3.5). Platelets and digested HA were then washed, and the HA remaining on the wells was detected colorimetrically. Activated platelets demonstrated significantly higher hyaluronidase activity (80% maximum) than nonactivated platelets (10% maximum) and platelet lysate (50% maximum). n = 3; ***P < .001. Maximum activity was achieved by HAase.
IBD patients have lower platelet HYAL2 protein and hyaluronidase activities than non-IBD controls
Multiple platelet abnormalities have been identified in patients with IBD, including increased numbers and levels of activation.35 In addition, proinflammatory HA is increased in the inflamed intestinal mucosa of IBD patients.10 We therefore hypothesized that platelet HYAL2 has a role in ECM-HA degradation in IBD and compared the levels of platelet HYAL2 and hyaluronidase activity in patients with IBD with those in non-IBD controls. The immunoblot assay and hyaluronidase assay we used were normalized to total protein because IBD patients are known to frequently have high platelet numbers and low platelet volume. We first analyzed HYAL2 levels in lysates of platelets from IBD patients (n = 17) and non-IBD controls (n = 13) and found that, interestingly, HYAL2 protein levels in IBD patient samples, on average, were 45% lower than in samples from non-IBD controls (P = .01). We then analyzed hyaluronidase activity in lysates of platelets from IBD patients (n = 8) and non-IBD controls (n = 8) and found that hyaluronidase activity in IBD patient samples was also significantly lower than samples from non-IBD controls (P < .001) (Figure 7B).
Platelets from IBD patients have lower HYAL2 and hyaluronidase activity than their non-IBD counterparts. Platelets collected from IBD patients and from healthy controls, as approved by the Cleveland Clinic Institutional Review Board, were washed and lysed. Total protein concentration of platelet lysates was determined by using Bradford assay. (A) Total protein (25 µg) from each sample was analyzed by immunoblotting for HYAL2. Densitometry analysis (ImageQuant TL, GE Healthcare Life Sciences, Fairfield, CT) showed that platelets from IBD patients (n = 17) displayed an average reduction of 45% in HYAL2 protein levels compared with non-IBD controls (n = 13; P = .01). (B) Platelet lysates containing 400 µg of total protein were analyzed for their hyaluronidase activity. Platelet lysates were incubated with purified HA immobilized to wells of a 96-well plate for 18 hours at 37°C (pH 3.5). Lysates and digested HA were then washed, and remaining HA on the wells was detected colorimetrically. IBD platelets demonstrated significantly lower hyaluronidase activity (n = 8) compared with their healthy counterparts (n = 8; P < .001). Data are presented as both a scatter plot showing mean and a box-and-whiskers plot showing median of 10 to 90 percentiles.
Platelets from IBD patients have lower HYAL2 and hyaluronidase activity than their non-IBD counterparts. Platelets collected from IBD patients and from healthy controls, as approved by the Cleveland Clinic Institutional Review Board, were washed and lysed. Total protein concentration of platelet lysates was determined by using Bradford assay. (A) Total protein (25 µg) from each sample was analyzed by immunoblotting for HYAL2. Densitometry analysis (ImageQuant TL, GE Healthcare Life Sciences, Fairfield, CT) showed that platelets from IBD patients (n = 17) displayed an average reduction of 45% in HYAL2 protein levels compared with non-IBD controls (n = 13; P = .01). (B) Platelet lysates containing 400 µg of total protein were analyzed for their hyaluronidase activity. Platelet lysates were incubated with purified HA immobilized to wells of a 96-well plate for 18 hours at 37°C (pH 3.5). Lysates and digested HA were then washed, and remaining HA on the wells was detected colorimetrically. IBD platelets demonstrated significantly lower hyaluronidase activity (n = 8) compared with their healthy counterparts (n = 8; P < .001). Data are presented as both a scatter plot showing mean and a box-and-whiskers plot showing median of 10 to 90 percentiles.
Discussion
In this article, we show that platelets degrade HA from the surfaces of activated human intestinal M-SMCs under physiological pH and that this degradation can be inhibited by blocking the activity of platelet HYAL2. We also demonstrate that platelet degradation of purified HA occurs only under acidic pH, consistent with previous reports on HYAL2 activity.32,36 Although HYAL2 is known to be a cell surface protein,37 we found little evidence of it on the surfaces of resting platelets. Instead, HYAL2 was detected exclusively on the surfaces of activated platelets. We also show for the first time that HYAL2 is packaged within α-granules in resting platelets, and that upon activation, HYAL2 is translocated from α-granules to the platelet surface, where it functions to digest HA. Finally, we show that patients with IBD have lower platelet HYAL2 levels and activity than healthy controls.
Our data raise several interesting questions. The observation that platelets degrade cell surface HA but not purified HA under physiological pH suggests that platelet-mediated HA degradation depends on multiple factors and is likely a regulated process. HYAL2 is reported to be an acid active enzyme.32 However, it was demonstrated in an embryonic kidney cell line that HYAL2 becomes active under physiological pH only when it coexists with CD44 at the plasma membrane.16 Another group has shown, in a breast cancer cell line, that the CD44-dependent HA degradation was achieved as a result of an increase in the activity of the sodium-hydrogen exchanger NHE1, causing a local drop in pH, which creates an acidic microenvironment for HYAL2.18 This is an unlikely scenario in platelets because intact platelets, either resting or activated, did not degrade purified HA under neutral pH. Additionally, there is no evidence that human platelets express CD44. In our laboratory, platelet degradation of HA was not inhibited when we pre-incubated platelets with anti-CD44 antibodies (data not shown). In our cell coculture model, a likely scenario is that HA-binding proteins (eg, versican, pentraxin 3, TSG6, and the heavy chains of inter-α-trypsin inhibitor)10,38,39 may be necessary to facilitate the interaction between platelets and HA and therefore mediate HA catabolism by platelets. This is similar to the requirements our group has noted for nonactivated leukocyte binding to HA cables.10 However, further research is needed to determine which HA-binding proteins may have a role in this reaction.
A second interesting point raised by our data is that HYAL2 is stored in a distinct subpopulation of α-granules, which lacks three known α-granule markers: P-selectin, vWF, and fibrinogen. Selective packaging of proteins within different subpopulations of α-granules in platelets has been a matter of controversy.40,41 For example, vascular endothelial growth factor, a proangiogenic protein, was shown to be stored in a different α-granule population than endostatin, an antiangiogenic protein.34 Conversely, it has been proposed that random distribution of proteins into α-granules occurs.42 However, the clear localization of HYAL2 within α-granules (observed by electron microscopy and sucrose density gradient analysis) and the complete lack of colocalization between HYAL2 and P-selectin (a protein thought to be present in all α-granules)34 favor the hypothesis that α-granule proteins can be separated into distinct subpopulations. Nonetheless, further research is needed to define the contents of the HYAL2-containing α-granules and to determine whether any functional coclustering of proteins within these granules exists.
Under healthy conditions, platelets do not adhere to the vascular wall. However, upon vascular injury, platelets are exposed to components of extracellular matrix (eg, collagen and fibronectin) to which they rapidly adhere.43 We previously reported that platelets can bind and degrade HA from the surfaces of activated endothelial cells.19 Here we show that HA in the extracellular matrix of polyI:C-treated M-SMCs can also be degraded by platelets. This is important because HA is upregulated during inflammation in many tissues, and smooth muscle cells and myofibroblasts are considered to be the major HA-producing cells. High levels of HA deposition have been reported in several inflammatory diseases including asthma, arthritis, and hepatitis.8,9,44,45 In addition, our group has reported an upregulation of HA in the intestines of patients with IBD, a chronic condition characterized by tissue erosion and intestinal bleeding.10,46
IBD is also associated with platelet dysfunction.35 Multiple platelet abnormalities have been identified in patients with IBD, including reactive thrombocytosis, low mean platelet volume, and increased density.47-49 Importantly, platelets in IBD patients circulate in the periphery in an increased activated state and are more susceptible to activation than platelets from healthy participants.50 Here, we present yet another abnormality associated with platelets from patients with IBD. Low platelet HYAL2 and hyaluronidase activity might have a role in the pathogenesis of IBD. One possibility is that the lack of HYAL2 in platelets might be contributing to intravascular microthrombi formation, a feature widely observed in IBD.51,52 This major abnormality in IBD mucosa is characterized by the presence of platelet thrombi cross-linked with fibrin in the mucosal microvasculature. Interestingly, it has been reported that HA can tightly bind fibrinogen53,54 ; in addition, there is a significant increase in HA deposition in tissues of IBD patients.10 Collectively, HYAL2 might be necessary to degrade HA and therefore prevent thrombus formation. Another mechanism by which HYAL2 might be contributing to IBD is its possible role in wound healing. Ulceration resulting from severe inflammation is a major feature in IBD and can cause damage to blood vessels. HA sharply accumulates during the inflammation phase of wound healing, and impaired clearance of HA may result in enduring inflammation.12,55 As a result, HA degradation by platelet HYAL2 might be crucial for proper tissue repair upon injury. Furthermore, HA fragments generated by platelet HYAL2 may have a role in improving wound healing.56 Both of these functions (the prevention of microthrombi formation and wound healing) could be impaired in IBD patients with low platelet HYAL2.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Judith Drazba for assistance with confocal microscopy, Mei Yin for assistance with electron microscopy, Gail West for assistance with cell isolation, A. Rocio Lopez for assistance with statistics, Dr Tony Calabro and Dr Thomas McIntyre for helpful conversations, and Dr Vincent Hascall for critical reading of the manuscript. S.A. is a PhD candidate at Cleveland State University and this work is submitted in partial fulfillment of the requirement for the PhD.
This work was supported by the Programs of Excellence in Glycosciences HL107147 from the National Heart, Lung, and Blood Institute (C.A.d.l.M.) and by startup funds from the Department of Pathobiology, Cleveland Clinic Lerner Research Institute (C.A.d.l.M.).
Authorship
Contribution: S.A. designed the research, performed experiments, analyzed results, and wrote the paper; K.A. performed experiments and analyzed results; D.R.H. performed experiments; B.S. provided IBD patient samples; and C.A.d.l.M. designed the research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carol A. de la Motte, Department of Pathobiology NC22, Cleveland Clinic Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: delamoc@ccf.org.