In this issue of Blood, Record et al report a novel human primary neutrophil immunodeficiency disorder caused by megakaryoblastic leukemia 1 (MKL1) mutation.1
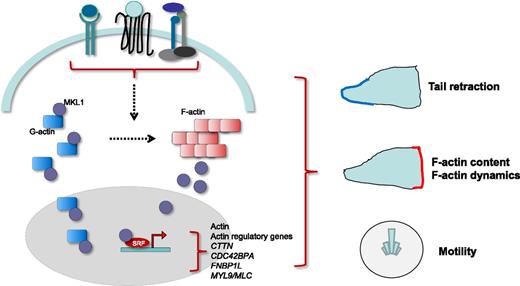
Actin dynamics are regulated by numerous stimuli engaging several plasma membrane receptors, including cytokine receptors, G-coupled chemokine receptors, and integrins. Molecules of G-actin sequester MKL1 into the cytoplasm. On stimulation, polymerization of G-actin into F-actin releases MKL1, which can translocate to the cytoplasm and bind nuclear transcription factor SRF. This stimulates expression of cytoskeletal genes, including actin itself and actin regulatory genes. Record et al show that MKL1 notably stimulates the expression of actin, CTTN, formin-binding protein FNBP1L, and myosin light chain (MYL9/MLC). MKL1, together with downstream targets, controls G- and F-actin content, F-actin dynamics, tail retraction, and cell motility.
Actin dynamics are regulated by numerous stimuli engaging several plasma membrane receptors, including cytokine receptors, G-coupled chemokine receptors, and integrins. Molecules of G-actin sequester MKL1 into the cytoplasm. On stimulation, polymerization of G-actin into F-actin releases MKL1, which can translocate to the cytoplasm and bind nuclear transcription factor SRF. This stimulates expression of cytoskeletal genes, including actin itself and actin regulatory genes. Record et al show that MKL1 notably stimulates the expression of actin, CTTN, formin-binding protein FNBP1L, and myosin light chain (MYL9/MLC). MKL1, together with downstream targets, controls G- and F-actin content, F-actin dynamics, tail retraction, and cell motility.
Polymorphonuclear neutrophils are the most abundant white blood cells in humans. They form the first line of cellular defense by rapidly migrating to the site of infection and killing invading microorganisms. These reactions are controlled by chemokine and integrin signaling pathways and necessitate actin cytoskeleton reorganization, degranulation, and oxidative burst. Any defects in neutrophil numbers or functions cause recurrent and life-threatening bacterial infections.2
Numerous inherited disorders of neutrophil functions have been identified, including leukocyte adhesion deficiencies, disorders of chemotaxis, chronic granulomatous disease, hyperimmunoglobulin E syndrome, Chédiak-Higashi syndrome, neutrophil-specific granule deficiency, and myeloperoxidase deficiency.2 Although rare, primary immune disorders affecting actin assembly have been identified, notably dominant-negative mutation in the small GTPase Rac2 gene3,4 and loss of function mutations in the Wiskott-Aldrich syndrome (WAS) gene,2 important regulators of actin dynamics. However, some neutrophil immunodeficiency syndromes are being diagnosed but remain undefined. In their study, Record et al1 describe a novel human primary immunodeficiency disorder: MKL1 mutation resulting in severe defects in actin rearrangement causing clinical presentation of neutrophil defects.
MKL1 and MKL2 are members of the myocardin-related transcription factor protein family.5 MKL1 was initially identified in patients with hematologic malignancies. MKL1 and MKL2 are widely expressed. They are coactivators of the nuclear transcription factor serum response factor (SRF). They are found in complex with globular actin (G-actin) in the cytoplasm, preventing their nuclear localization. Incorporation of G-actin into filamentous actin (F-actin) in response to stimuli liberates MKL1/2, enabling their nuclear translocation and interaction with SRF. This stimulates expression of cytoskeletal genes, including actin and actin regulatory genes (see figure). Nuclear MKL can bind nuclear G-actin, subsequently inhibiting SRF-driven gene expression, providing feedback regulation. Thereby, the actin-MKL-SRF circuit modulates gene expression along with cytoskeletal dynamics for the regulation of cell motility as well as cell survival, proliferation, and differentiation.5 MKL2 and myocardin knockout mice are embryonic lethal.5 MKL1 knockout mice are viable with impaired megakaryopoiesis, causing reduced platelet counts in peripheral blood.6 Surprisingly, the role of MKL1 has remained elusive in immune cells.
Record et al describe an MKL1 mutation in a patient who presented with clinical symptoms suggestive of neutrophil function defects. In the first year of life, the patient developed Pseudomonas septic shock associated with meningitis, malignant otitis externa, and numerous cutaneous and subcutaneous abscesses. Although she recovered, she continued to have intermittent deep skin and ear infections and failed to thrive. Patients with neutrophil function defects exhibited impaired phagocytosis and profound migratory defects. Interestingly, patient neutrophils which did migrate exhibited a unique phenotype with reduced velocity but increased directional persistence. This abnormal migratory behavior raised the question of defects in actin dynamics. As such, patient neutrophils exhibited a reduction in G-actin and F-actin content. The MKL1 mutation also causes a marked reduction in F-actin in dendritic cells, B cells, and fibroblasts.
The MKL1 mutation described here results in the absence of full-length MKL1 protein expression. The authors use short hairpin-mediated MKL1 knockdown in the human neutrophilic cell line HL60 to validate MKL1 function in human neutrophils. Silencing MKL1 in HL60-derived neutrophils remarkably mimics the patient neutrophil phenotype, with impaired neutrophil chemotaxis associated with enhanced directional persistence.
Although the MKL1 mechanism of action was not fully investigated, additional experiments provide important insights into how MKL1 deficiency might cause defective neutrophil functions. G- and F-actin neutrophil contents were drastically reduced in MKL1 knockdown HL60 cells. Interestingly, restoration of G-actin expression in these cells only partially restored F-actin content, suggesting MKL1 plays a nonredundant role in controlling expression of actin genes, as perhaps anticipated. It also implies other MKL1 functions in actin polymerization regulation. Gene expression analysis reveals that MKL1 knockdown reduces expression of several regulators of actin dynamics, including CDC42BPA, CTTN, FNBP1L, and MYL9/MLC (see figure). Although their causal roles in the MKL1 knockdown phenotype were not examined, reduced expression of cortactin (CTTN) and formin-binding protein FNBP1L may contribute to defective actin dynamics. FNBP1L coordinates actin assembly into lamellipodia by binding to WAS protein (WASp). FNBP1L colocalizes with cortactin into F-actin–rich structures.7
Another interesting observation is the abnormal uropod extension exhibited by MLK1-deficient HL60-derived neutrophils. Motility is controlled by coordination of adhesion and deadhesion to propel the cell body forward. A long uropod reflects a lack of deadhesion due to strengthened integrin functions. This is controlled by the RhoA-ROCK-MLC (Ras homolog family member A–rho-associated, coiled-coil containing protein kinase 1–myosin light chain) pathway.8 Hence, a likely result of lack of MLC expression would be impaired tail detachment, which may directly contribute to the persistence migratory behavior of these cells. Interestingly, as with MKL knockdown, SRF-deficient neutrophils exhibited impaired F-actin assembly and expression of actin regulatory genes.9 SRF plays an unexpected role in integrin internalization. It would be interesting to investigate the role of MKL1 in integrin regulation.
As shown in this study, MKL1 is important for cytoskeletal regulation in various cell types, including myeloid lineages, lymphoid cells, and fibroblasts in vitro. It also controls megakaryopoiesis in mice.6 However, the major clinical presentation of MKL1 mutation appears to relate to neutrophil immunodeficiency. This may reflect the importance of neutrophils for antibacterial defenses. Alternatively, neutrophils may be specifically dependent on this pathway for optimal functioning in vivo. Although ubiquitous, the molecular machinery of actin dynamics is highly cell-type specific. As with MKL1 deficiency, a loss-of-function mutation in WASp causes severe defects in cytoskeleton regulation in myeloid cells, lymphoid cells, and platelets. However, bleeding and clinical manifestations of lymphocyte defects are more prominent in WAS patients.2 The authors may have uncovered a regulatory circuit of actin dynamics that is uniquely critical for neutrophil functions. It will be important to investigate in detail how MKL1 is regulated during infections and how it controls neutrophil functions.
This study underscores the need for screening for gene mutations in immunodeficient patients. This is important from the perspective of basic science to uncover new regulatory pathways of neutrophil functions. Clinically, the identification of specific mutations responsible for inherited immunodeficiency disorders offers alternative therapeutic options, such as gene therapy, in the absence of matched donors for stem cell transplantation. In the era of gene-editing technologies, personalized medicine may become a therapeutic choice.
Conflict-of-interest disclosure: The author declares no competing financial interests.