Elusive CD8+ T cells that transiently secrete interleukin (IL)-17 cause graft-versus-host disease (GVHD) but do not contribute to beneficial graft-versus-leukemia (GVL) responses, as reported by Gartlan et al in this issue of Blood.1 GVHD remains a lethal and morbid complication of allogeneic bone marrow transplantation, but GVHD is tightly linked to beneficial GVL effects, and removal of donor T cells that cause GVHD also diminish GVL, leading to greater relapse after bone marrow transplantation (BMT). This elegant paper from the laboratory of Geoffrey Hill has identified a rare “night fury” T-cell subset that causes much pain with no gain, a finding that may take us one large step closer to the long sought after goal of separating GVL and GVHD.
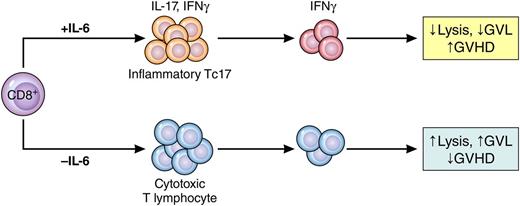
Phantom Tc17 cells mediate GVHD but not GVL. Under the influence of IL-6, CD8+ T cells differentiate into weakly cytolytic lymphocytes that secrete IFN-γ but only transiently secrete IL-17 (Tc17). These cells cause GVHD but do not mediate GVL effects. When IL-6 is absent, CD8+ T cells differentiate into potent cytolytic T lymphocytes that mediate GVL but do not cause GVHD. Professional illustration by Patrick Lane, ScEYEnce Studios.
Phantom Tc17 cells mediate GVHD but not GVL. Under the influence of IL-6, CD8+ T cells differentiate into weakly cytolytic lymphocytes that secrete IFN-γ but only transiently secrete IL-17 (Tc17). These cells cause GVHD but do not mediate GVL effects. When IL-6 is absent, CD8+ T cells differentiate into potent cytolytic T lymphocytes that mediate GVL but do not cause GVHD. Professional illustration by Patrick Lane, ScEYEnce Studios.
Cytolytic T cells (CTLs) that mediate GVL effects are predominantly CD8+, and therefore, elimination of the entire CD8+ T-cell subset usually leads to greater relapse. Current GVHD prophylaxis efforts, such as calcineurin inhibitors or antithymocyte globulin, are nonspecific and target all T cells. It has not been clear which donor T-cell subpopulations could be eliminated without impairing GVL effects or damaging reconstitution of the patient’s immune system after BMT. The Hill group had previously reported that IL-17, an inflammatory cytokine that is important in autoimmune disease, also mediates GVHD in experimental models.2 Here they identified a weakly cytolytic but highly inflammatory subset of CD8+ cells whose removal costs nothing in terms of GVL activity but virtually eliminates lethal GVHD. Because the functions of many T cells are not firmly fixed when they first encounter their targets, these functions can alter during rapid clonal expansion. The authors therefore tracked their quarry using an elegant fate-mapping technique and identified phantom CD8+ cells that secrete IL-17 for only a brief period of time but continue to secrete other cytokines such as interferon (IFN)-γ (see figure).
The IL-17 axis is complex and important, particularly as it relates to GVHD in the gastrointestinal tract, which is the target organ most resistant to therapy and most likely to give rise to transplant-related mortality. Subpopulations of T cells that produce IL-17 have already been identified: one is pathogenic and secretes IFN-γ and the other other is nonpathogenic and secretes IL-10.2 Tc17 cells also produce IL-22, as the authors demonstrate, but IL-22 can protect intestinal stem cells and can regulate GVHD.3 The story is further complicated by the fact that excess IL-17 can decrease the production of IL-22, disturbing gastrointestinal barrier function, which can be reversed with endogenous IL-22.4 The IL-17/IL-22 axis also induces regenerative 3 α, a Paneth cell protein that is a validated biomarker of gastrointestinal GVHD.5 Thus, IL-17 after BMT is key to the reconstitution of mucosal immunity and the restoration of normal barrier function after BMT, but in excess, it is primarily inflammatory, even for short periods, and can cause severe gastrointestinal damage.
It should be noted that it is not yet clear that these cells are present in clinical GVHD, and given their elusive nature and the morbidity of liver and gastrointestinal biopsies early after BMT in thrombocytopenic and neutropenic patients, it will likely be exceedingly difficult to demonstrate them. Nevertheless, strategies that may be able to eliminate them are already being tested in the clinic. The authors show that IL-6 is a key inducer of inflammatory Tc17 cells (as expected, because Tc17 cells are known to depend on IL-6), and the blockade of IL-6 after BMT prevents their proliferation and subsequent GVHD damage. Recently, an anti–IL-6 receptor monoclonal antibody, tocilizumab, was tested as GVHD prophylaxis in a small clinical trial of 48 patients.6 The overall incidence of grade 2 to 4 GVHD was 12% and that of grade 3 to 4 GVHD was only 4%. Relapse of leukemia was within the expected range: ∼25% at 2 years. This study provides a strong rationale as to why the use of anti–IL-6 is unlikely to increase the risk of relapse even though it significantly reduces GVHD and engenders enthusiasm for larger definitive trials of this approach.
Conflict-of-interest disclosure: The author declares no competing financial interests.