Key Points
Severe CPN in adults is a benign entity without secondary myeloid malignancies.
Neutrophil count at diagnosis is the only predictive factor of severe infections.
Abstract
Severe chronic primary neutropenia (CPN) is a rare entity, and long-term outcome and risk factors for infections in severe CPN adults have not been described to date. We report the characteristics and outcomes of 108 severe adult CPN patients enrolled in a multi-institutional observational study. Severe CPN adults were mostly female (78%), and median age at diagnosis was 28.3 years. Diagnosis was fortuitous in 62% of cases. The median absolute neutrophil count (ANC) at diagnosis was 0.4 × 109/L, and median ANC without granulocyte colony-stimulating factor (G-CSF) during follow-up was 0.5 × 109/L. Twenty-three of 66 (34.8%) evaluable patients had neutrophil autoantibodies, and 6 of 47 (12.8%) a T-cell clone. The presence of neutrophil autoantibodies or T-cell clone was not associated with any specific clinical or biological characteristics. No death or hematologic malignancies occurred, and 44 severe bacterial infections were reported in 27 patients with a median follow-up of 8.3 years. Fifty patients received G-CSF either sporadically (n = 24) or continuously (n = 26) and responded (96%). Nineteen patients received immunosuppressive therapies: overall response (OR) was 41%, and median duration of response was 3 months. At diagnosis, the only predictive factor for the occurrence of severe bacterial infections was an ANC count below 0.2 × 109/L (OR, 0.76). Severe CPN in adults is characterized by a female predominance and a benign outcome with a low rate of severe bacterial infections and no secondary malignancies. G-CSF is efficient and well tolerated but is not required in a majority of patients.
Introduction
Severe chronic idiopathic neutropenia (CIN) was described in 1968 and is defined by persistent neutrophil counts below 0.5 × 109/L, in the absence of an identifiable etiology.1 Severe chronic primary neutropenia (CPN) in adults includes true idiopathic neutropenia (CIN), primary autoimmune neutropenia defined by the presence of neutrophil auto-antibodies, and CPN associated with a T-cell clone (excluding large granular lymphocyte leukemia).2-10 So far, there is no evidence based on clinical characteristics and outcome to consider these entities as different.
Among the 374 adults enrolled in the Severe Chronic Neutropenia International Registry (35 countries), 224 (60%) corresponded to CPN.11,12 However, these patients were not reported in detail. Only small, unicentric studies of nonsevere CPN and case reports have been published to date (briefly detailed in supplemental Table 1 available on the Blood Web site).1,4,5,7,11-16 All these studies reported a favorable outcome and suggested the heterogeneity of this condition. Epidemiological, clinical, and biological characteristics; incidence of severe infections; and treatment efficacy have not been reported so far in a large cohort of adults with severe CPN.
We report here on 108 adult patients with severe CPN followed for a median of 8.3 years and enrolled in the French Severe Chronic Neutropenia Registry. Individual risk factors at diagnosis were analyzed for their associations with the development of severe bacterial infections during follow-up. The impact of granulocyte–colony-stimulating factor (G-CSF) therapy on severe infections was evaluated.
Patients and methods
All patients were prospectively enrolled in the French Severe Chronic Neutropenia Registry, which was established in 1993 and aimed to include all patients with severe chronic neutropenia of any type.17 The registry received national certification in 2008. A clinical research associate (B.B.) performed medical records collection yearly in centers, and 2 physicians reviewed the data (F.S.d.F. and J.D.). Patients or their legal guardians provided written informed consent at inclusion in the registry. The institutional review board approved this study.
Patients were included in the present study if they met the following criteria: (1) absolute neutrophil count (ANC) below 0.5 × 109/L or below 1 × 109/L but with severe/recurrent infections and/or chronic aphtous stomatitis, during a period of at least 3 months; (2) follow-up of at least 1 year from the first low ANC; (3) normal hemoglobin and platelet counts at diagnosis; (4) a bone marrow cytological examination excluding hematologic malignancies; (5) normal bone marrow cytogenetics if performed; and (6) informed consent to the inclusion on the registry. Exclusion criteria were (1) prior cytotoxic treatment; (2) hematologic malignancy (mainly myelodysplasia, lymphoproliferative disorders including large granular lymphocyte leukemia); (3) conditions known to be associated with neutropenia (rheumatoid arthritis or Felty syndrome, systemic lupus erythematous, Goujerot Sjogren syndrome); (4) immunodeficiency or associated disorder; (5) a history of congenital disorder with other organ dysfunction or a family history of cytopenia; (6) a first low neutrophil count before the age of 15; and (7) a follow-up shorter than 1 year.
Recorded data included demographic information, hematologic and immunologic parameters, infectious and noninfectious manifestations, and therapeutic history. Neutropenia was considered as moderate, severe, or very severe when ANCs were lower than 1 × 109/L, 0.5 × 109/L, and 0.2 × 109/L, respectively.
Neutropenia was considered to be permanent if the ANC remained below the normal value throughout the study period and fluctuating when normal ANCs were observed occasionally during the study period. Spontaneous recovery of CPN was considered if the neutropenia resolved (ANC higher than 1.7 × 109/L) without any treatment with a follow-up of at least 2 years after recovery.
Bacterial infections were considered severe if they required intravenous antibiotics and hospitalization (ie, severe sepsis, abscess, pneumonia). Fever of unknown origin was not considered as bacterial infection. Aphtous stomatitis corresponded to severe, recurrent oral ulcers. Hypergammaglobulinemia was defined as a gammaglobulin level above 15 g/L on serum protein electrophoresis and/or an immunoglobulin (Ig) G level above 13 g/L. Hypogammaglobulinemia was defined as a gammaglobulin level below 7 g/L on serum protein electrophoresis and/or an IgG level below 6 g/L. Lymphopenia was defined as lymphocyte counts below 1 × 109/L on more than 2 evaluations in the absence of any treatment. Monocytopenia and monocytosis were defined as monocyte counts below 0.2 × 109/L and above 1.2 × 109/L respectively. Anti-nuclear antibodies (ANAs) were considered to be positive if the titer was above 1/100. For other antibodies, the laboratory reference values were considered. Patients with autoantibodies above the threshold and related clinical symptoms were excluded. Anti-granulocyte antibodies were diagnosed based on GAT, GIFT, and MAIGA as recommended.18,19 Biological autoimmunity was defined as a composite score with a value of 1 if the patient had at least 1 autoantibody at significant levels excluding anti-granulocyte antibodies, and 0 otherwise. G-CSF therapy was considered to be planned if G-CSF was administrated on a regular basis and to be sporadic if it was administrated only according to clinical or hematologic symptoms. Efficacy of G-CSF and immunosuppressive therapies was evaluated according to the ANC (>1 × 109/L) and the resolution of symptoms.
Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR) and were compared using the Wilcoxon rank sum test. Categorical variables were compared using the χ2 test. Follow-up was defined as the interval between diagnosis and last visit. Patients were censored at date of last visit. Patients were considered lost to follow-up if the date of last visit exceeded 5 years from June 30, 2014 (cutoff date for the analysis). Cumulative incidences of severe bacterial infections were compared as previously described.20 The influence of clinical and biological factors at diagnosis on the occurrence of severe bacterial infections was analyzed by logistic regression. Statistical analyses were performed using R software version 3.0 (http://cran.r-project.org). Statistical significance was considered as P = .05, and all tests were 2-sided.
Results
Demography
Overall the charts from 231 patients were reviewed, and 123 patients were excluded. Causes of exclusion are detailed in supplemental Table 2. One hundred eight patients were included. Eighty-five (78.7%) were female (sex ratio, female/male = 3.7). The median age at diagnosis was 28.3 years [range 21.5-39.9]. The diagnosis was fortuitous in 67 patients (62%), or made in the setting of a severe bacterial infection in 5 (4.6%), a nonsevere infection in 25 (23.1%), or recurrent aphtous stomatitis in 11 (10.2%) cases.
Median follow-up was 8.3 years accounting for 1143 patient-years of follow-up. Spontaneous complete remission was observed in 2 patients after 3.8 and 11.9 years of follow-up, respectively. Eighteen patients (16.7%) were lost to follow-up after a median time of 12 years (4.1-18.4).
Hematologic and immunologic parameters
The median ANC at diagnosis was 0.41 × 109/L [0.29-0.65]. The median of all ANC counts evaluated without G-CSF for each patient was 0.5 × 109/L [0.38-0.67]. Twenty-eight (26%), 72 (67%), and 8 (7%) patients were classified as having very severe, severe, and moderate CIN, respectively. Thirty-four patients (31.5%) presented with lymphopenia. Eight (7.4%) patients had monocytopenia, and 5 (4.6%) monocytosis. Monocytopenia and lymphopenia were present concomitantly in 4 patients (3.5%). Clinical, hematologic, and immunologic characteristics are detailed in Tables 1 and 2, and in supplemental Table 3 for lymphocyte subpopulations.
Patients and CPN characteristics at diagnosis
| . | All patients, N = 108 . |
|---|---|
| Female (%) | 85 (78.7) |
| Age at diagnosis in years, median [IQR] | 28.3 [21.5-39.8] |
| CPN severity (%) | |
| Moderate (0.5-1 × 109/L) | 8 (7.4) |
| Severe (0.2-0.5 × 109/L) | 72 (66.7) |
| Very severe (<0.2 × 109/L) | 28 (25.9) |
| ANC at diagnosis (109/L), median [IQR] | 0.41 [0.29-0.65] |
| Neutrophil count without G-CSF during the study period (109/L), median [IQR] | 0.5 [0.39-0.67] |
| Minimum neutrophil count (109/L), median [IQR] | 0.18 [0.09-0.30] |
| Number of available complete blood counts per patient, median [IQR] | 10 [7-21] |
| Fluctuating neutropenia (%) | 24 (22.2) |
| Diagnostic circumstances (%) | |
| Fortuitous | 67 (62) |
| Banal infection | 25 (23.1) |
| Severe infection | 5 (4.1) |
| Aphtous stomatitis | 11 (10.2) |
| . | All patients, N = 108 . |
|---|---|
| Female (%) | 85 (78.7) |
| Age at diagnosis in years, median [IQR] | 28.3 [21.5-39.8] |
| CPN severity (%) | |
| Moderate (0.5-1 × 109/L) | 8 (7.4) |
| Severe (0.2-0.5 × 109/L) | 72 (66.7) |
| Very severe (<0.2 × 109/L) | 28 (25.9) |
| ANC at diagnosis (109/L), median [IQR] | 0.41 [0.29-0.65] |
| Neutrophil count without G-CSF during the study period (109/L), median [IQR] | 0.5 [0.39-0.67] |
| Minimum neutrophil count (109/L), median [IQR] | 0.18 [0.09-0.30] |
| Number of available complete blood counts per patient, median [IQR] | 10 [7-21] |
| Fluctuating neutropenia (%) | 24 (22.2) |
| Diagnostic circumstances (%) | |
| Fortuitous | 67 (62) |
| Banal infection | 25 (23.1) |
| Severe infection | 5 (4.1) |
| Aphtous stomatitis | 11 (10.2) |
Hematologic and immunologic characteristics
| . | Positive/evaluable patients (%) . |
|---|---|
| Bone marrow examination | |
| Normal | 37/108 (34.3) |
| Granular hypoplasia | 16/108 (14.8) |
| Late maturation arrest | 33/108 (30.6) |
| Increase cellularity | 22/108 (20.3) |
| Marrow cytogenetic analysis available | 60/108 (55.6) |
| Lymphocyte counts (109/L), median [IQR] | 1.4 [1.11-1.9] |
| Lymphopenia | 34/108 (31.5) |
| Monocytopenia | 8/108 (7.4) |
| Monocytosis | 5/108 (4.6) |
| CD4/CD8 ratio | 1.52 [1.21-2.10] |
| Gammaglobulin level (g/L), median [IQR] | 10.9 [9.9-13.1] |
| IgG level (g/L), median [IQR] | 11.8 [9.6-13.2] |
| Hypergammaglobulinemia (%) | 28/96 (29.2) |
| Neutrophil autoantibodies | 23/66 (34.8) |
| CD16 specificity | 10/23 (43.5) |
| Unknown specificity | 13/23 (56.5) |
| T-cell clone (%) | 6/47 (12.8) |
| Biological autoimmunity | |
| ANAs | 20/82 (24.1) |
| Anti-dsDNA | 9/62 (14.3) |
| Anti-SSA/Ro | 1/45 (2.2) |
| Anti-SSB/Lo | 0/42 (0) |
| Rheumatoid factor | 10/64 (15.4) |
| ANCA | 4/22 (17.4) |
| DAT | 0/31 (0) |
| Other autoantibodies present | 9/108 (8) |
| Overall (at least 1 positive) | 39/83 (47) |
| . | Positive/evaluable patients (%) . |
|---|---|
| Bone marrow examination | |
| Normal | 37/108 (34.3) |
| Granular hypoplasia | 16/108 (14.8) |
| Late maturation arrest | 33/108 (30.6) |
| Increase cellularity | 22/108 (20.3) |
| Marrow cytogenetic analysis available | 60/108 (55.6) |
| Lymphocyte counts (109/L), median [IQR] | 1.4 [1.11-1.9] |
| Lymphopenia | 34/108 (31.5) |
| Monocytopenia | 8/108 (7.4) |
| Monocytosis | 5/108 (4.6) |
| CD4/CD8 ratio | 1.52 [1.21-2.10] |
| Gammaglobulin level (g/L), median [IQR] | 10.9 [9.9-13.1] |
| IgG level (g/L), median [IQR] | 11.8 [9.6-13.2] |
| Hypergammaglobulinemia (%) | 28/96 (29.2) |
| Neutrophil autoantibodies | 23/66 (34.8) |
| CD16 specificity | 10/23 (43.5) |
| Unknown specificity | 13/23 (56.5) |
| T-cell clone (%) | 6/47 (12.8) |
| Biological autoimmunity | |
| ANAs | 20/82 (24.1) |
| Anti-dsDNA | 9/62 (14.3) |
| Anti-SSA/Ro | 1/45 (2.2) |
| Anti-SSB/Lo | 0/42 (0) |
| Rheumatoid factor | 10/64 (15.4) |
| ANCA | 4/22 (17.4) |
| DAT | 0/31 (0) |
| Other autoantibodies present | 9/108 (8) |
| Overall (at least 1 positive) | 39/83 (47) |
Anti-SSA/Ro, anti-Sjogren's-related antigen A/Ro; anti-SSB/La, anti-Sjogren's-related antigen B/Lupus La; ANCA, antineutrophil cytoplasmic antibodies; DAT, direct antiglobulin test
Thirty-seven (34.3%) patients had a normal bone marrow smear. Sixteen (14.8%) had granular hypoplasia; 33 (30.6%), a late maturation arrest; and 22 (20.4%), an increased cellularity. Cytogenetic analysis was available in 60 patients (55.6%) and was normal for all.
Hypergammaglobulinemia was present in 30%. Autoantibodies other than neutrophil antibodies were detected in 39 of 83 evaluable patients (47%) and are detailed in Table 2. ANAs, anti–double-stranded DNA (dsDNA) antibodies, and rheumatoid factor were the more common markers of biological autoimmunity. Neutrophil antibodies were evaluated in 66 patients and were positive in 23 (34.8%). The target antigen specificity was CD16 for 10 patients and was not determined for 13 patients. A clonal rearrangement of the T-cell receptor genes was identified in the blood of 6 of the 47 patients tested (12.8%). None of the 55 patients evaluated had ELANE mutations. Additionally, 12 and 8 of these patients were tested for GATA2 and CXCR4 mutations and were negative.
Clinical presentation and outcome
Recurrent aphtous stomatitis was reported in 49 patients (45.4%). Twenty-two (20.4%) patients had other clinical features, mainly including a history of thyroiditis (n = 9) and unexplained chronic arthralgia (n = 5; Table 3).
Infectious and noninfectious complications observed during follow-up
| . | All patients, n = 108 . |
|---|---|
| Patients with infectious complications | n (%) |
| Bacterial infections | 65/108 (60.2%) |
| Severe bacterial infections | 27/108 (25%) |
| Invasive fungal infections | 1/108 (0.9%) |
| Aphtous stomatitis | 49/108 (45.4%) |
| Associated features | 22/108 (20.4%) |
| Thyroiditis | 9/22 |
| Arthralgia | 5/22 |
| Cutaneous lupus | 2/22 |
| Vitiligo | 1/22 |
| Myelitis | 1/22 |
| Granulomatosis | 1/22 |
| Others | 3/22 |
| . | All patients, n = 108 . |
|---|---|
| Patients with infectious complications | n (%) |
| Bacterial infections | 65/108 (60.2%) |
| Severe bacterial infections | 27/108 (25%) |
| Invasive fungal infections | 1/108 (0.9%) |
| Aphtous stomatitis | 49/108 (45.4%) |
| Associated features | 22/108 (20.4%) |
| Thyroiditis | 9/22 |
| Arthralgia | 5/22 |
| Cutaneous lupus | 2/22 |
| Vitiligo | 1/22 |
| Myelitis | 1/22 |
| Granulomatosis | 1/22 |
| Others | 3/22 |
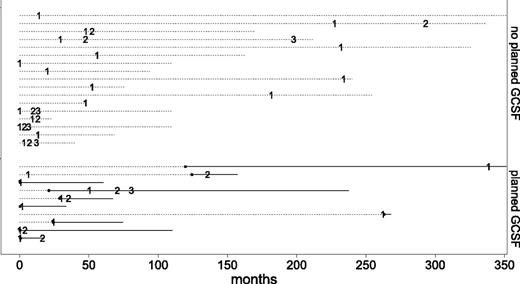
Bacterial infections were reported in 65 (60.2%) patients (Table 3). Twenty-seven (25%) patients developed 44 severe bacterial infections giving an incidence rate of 3.85 infections per 100 patient-years. Severe bacterial infections are detailed in supplemental Table 4. Fifteen patients presented 1 episode of severe bacterial infection; 7 patients, 2 episodes; and 5 patients, 3 episodes. The median time from diagnosis to first severe bacterial infection was 24.3 months [2.45-54]; the chronology of severe bacterial infections is depicted in Figure 1. One patient experienced a fungal infection (invasive aspergillosis) after a treatment with antithymocyte globulin, ciclosporine, and methylprednisone.
Chronology of severe bacterial infections in the 27 CPN patients. Numbers represent the episode of severe bacterial infection (first, second, and third). Dashed lines represent the follow-up for each patient. Solid lines represent the duration of G-CSF for patients who received planned G-CSF therapy. Filled dots represent initiation of planned G-CSF therapy in these patients.
Chronology of severe bacterial infections in the 27 CPN patients. Numbers represent the episode of severe bacterial infection (first, second, and third). Dashed lines represent the follow-up for each patient. Solid lines represent the duration of G-CSF for patients who received planned G-CSF therapy. Filled dots represent initiation of planned G-CSF therapy in these patients.
Medical management
Overall, 54 patients (50%) received a treatment of neutropenia (Table 4). The median time from diagnosis of CPN to first treatment was 23.1 months (3.8-85.2). Fifty patients received G-CSF. Twenty-four patients (22.2%) received G-CSF regularly as part of their treatment plan (planned G-CSF), whereas 26 (24.1%) received G-CSF sporadically only in case of infection or decreasing ANC. Median duration of G-CSF treatment was 1415 days [1032-2762] and 111 days [22-464] in the planned and sporadic groups, respectively. The median dose of G-CSF in the 23 responders in the planned G-CSF group was 4.5 µg/kg per week [2.8-6.4].
G-CSF and immunosuppressive treatments in CPN patients
| . | All patients, n = 108 . |
|---|---|
| Treatment (%) | 54 (47.4%) |
| Treatment indication | |
| Nonsevere bacterial infection | 18/54 (33.3%) |
| Severe bacterial infection | 15/54 (27.8%) |
| Aphtous stomatitis | 13/54 (24.1%) |
| Neutropenia | 7/54 (13%) |
| Asthenia | 1/54 (1.9%) |
| Planned G-CSF treatment | 24/50 (48%) |
| Median duration (days), [IQR] | 1415 [1032-2762] |
| Complete response to G-CSF (%) | 23/24 (96) |
| Sporadic G-CSF treatment | 26/50 (52%) |
| Mediation duration (days), [IQR] | 111 [22-464] |
| Other treatments | 19/54 |
| Response to immunosuppressive treatments | 8/19 (44.4%) |
| Number of immunosuppressive treatments | |
| 1 | 9/19 (47.4%) |
| 2 | 6/19 (31.6%) |
| ≥3 | 4/19 (20%) |
| . | All patients, n = 108 . |
|---|---|
| Treatment (%) | 54 (47.4%) |
| Treatment indication | |
| Nonsevere bacterial infection | 18/54 (33.3%) |
| Severe bacterial infection | 15/54 (27.8%) |
| Aphtous stomatitis | 13/54 (24.1%) |
| Neutropenia | 7/54 (13%) |
| Asthenia | 1/54 (1.9%) |
| Planned G-CSF treatment | 24/50 (48%) |
| Median duration (days), [IQR] | 1415 [1032-2762] |
| Complete response to G-CSF (%) | 23/24 (96) |
| Sporadic G-CSF treatment | 26/50 (52%) |
| Mediation duration (days), [IQR] | 111 [22-464] |
| Other treatments | 19/54 |
| Response to immunosuppressive treatments | 8/19 (44.4%) |
| Number of immunosuppressive treatments | |
| 1 | 9/19 (47.4%) |
| 2 | 6/19 (31.6%) |
| ≥3 | 4/19 (20%) |
G-CSF was clinically effective in 43 patients: 23 of 24 (96%) and 20 of 26 (80%) in the planned and sporadic G-CSF groups, respectively. In the planned G-CSF group, the median ANC count increased from 0.59 × 109/L [0.35-0.8] to 2.06 × 109/L [1-6.07] after treatment in the 23 responding patients. Tolerance of G-CSF was good: 17 of 50 patients reported mild adverse effects (bone pain in 14 and headaches in 5) that were efficiently managed by lowering G-CSF doses. Nineteen patients received an immunosuppressive therapy for neutropenia (details in supplemental Table 5); 4 of them did not receive G-CSF. Response to the most commonly used immunosuppressive treatments (prednisolone, methotrexate, and cyclosporine) was observed in half of the patients but was mostly partial and transient, with a secondary loss of response after tapering or treatment discontinuation. No patient died or developed a hematologic malignancy during the study period.
A transient profound asymptomatic neutropenia was detected at birth in 4 infants of 4 women with CPN (2 girls and 2 boys; nadir was observed in the first days of life with an ANC of 0.07, 0.05, 0.3, and 0.05 × 109/L). Evolution was spontaneously favorable in all after 2 to 6 months. Only 1 of the 3 mothers tested had neutrophil autoantibodies of unknown significance. All were also negative for ANA, ANCA, and anti-dsDNA antibodies. Neutrophil antibody evaluation was negative for 2 of the infants.
Determinant of severe bacterial infections
Age and gender did not impact the clinical or biological characteristics of CPN. Monocytopenia and very severe CPN were both associated with bone marrow granular hypoplasia. Monocytopenia was present in 5 of 16 (31%) and 3 of 92 (3%) of patients with and without bone marrow granular hypoplasia, respectively (P = .001). Similarly, granular hypoplasia was significantly associated with very severe CPN (50% of patients with granular hypoplasia vs 22% of patients without, P = .04). Patients with neutrophil antibodies (23 patients) were more frequently symptomatic at diagnosis (presence of infections or aphtous stomatitis) compared with those without antibodies (65% vs 33%, P = .034). There were no differences between patients with or without a peripheral T-cell clone in demographic, hematologic, or immunologic parameters.
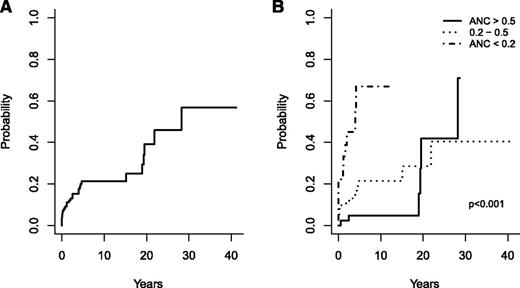
The only predictive factor for occurrence of severe bacterial infections during follow-up was the ANC at diagnosis with an overall response of 0.76 (95% confidence interval [CI], 0.65-0.87). The cumulative incidence of severe bacterial infections was 3.7% in the year following diagnosis, 21% at 5 and 10 years, and 39.1% at 20 years (Figure 2A-B).
Cumulative incidence of severe bacterial infections. Incidence in the whole cohort (A) and according to the severity of neutropenia at diagnosis (B).
Cumulative incidence of severe bacterial infections. Incidence in the whole cohort (A) and according to the severity of neutropenia at diagnosis (B).
Sixteen episodes of severe bacterial infections developed among the 24 patients who received planned G-CSF, 9 before the initiation of G-CSF, and 7 after, giving an adjusted incidence rate of severe bacterial infection of 7.5 per 100 patient-years [95% CI, 4-14] before and 3.9 per 100 patient-years [95% CI, 1.8-8] after G-CSF treatment, respectively (P = .2).
Discussion
Severe CPN is a heterogeneous entity first described in 1968.1 Most of the previously published studies included both severe and nonsevere cases (ANC ranging from <0.2 to 2 × 109/L).4,5,7,8,14,16 Moreover, primary and secondary neutropenia were often pooled in small and mostly unicentric studies. We report here the clinical, biological, and long-term outcome of the largest severe CPN cohort in adults followed for a median of 8.3 years. Despite a median ANC of 0.5 × 109/L (0.39-0.67) throughout the observation period, severe infections were rare, and patients had an overall favorable outcome without specific mortality.
We observed a clear female predominance (80%), in agreement with previously reported CPN.1,14 The median age at diagnosis of 28 years in this cohort was younger than previously reported (41 years in the 15 patients described by Kyle and Linman).1
The diagnosis was fortuitous in 62% of the patients. Twenty-two percent of the patients had fluctuations of their neutrophil counts over time, from very low counts to normal values without evidence of cyclic neutropenia. Similar fluctuations of ANC have been underlined in the first reported CPN cases by Kyle and Linman.1 Only 2 patients experienced complete prolonged remission of the disease.
We could not perform a centralized review of the bone marrow smears in our study; however, detailed reports were available for all patients. The bone marrow features were heterogeneous with 34% of the patients having normal marrow, 31% having late maturation arrest, 20% having hypercellular marrow, and 15% having granular hypoplasia. This heterogeneity is similar to that found in the 49 patients with primary CPN reported by van der Veen et al despite different inclusion criteria (with notably less severe CPN).14 Kyle and Linman excluded patients with granular hypoplasia from his initial study.1 In our study, granular hypoplasia was associated with monocytopenia and lower ANC at diagnosis; however, no other difference in presentation and outcome was found, suggesting that it does not represent a distinct entity. A subset of our patients presented with late maturation arrest in their bone marrow evaluation, a pattern previously described by Kyle and Linman.1 Whether this cytological pattern represents a characteristic feature indicative of a terminal maturation defect or an increased marrow release in some cases of CPN is not known. Overall, a typical marrow appearance in CPN could not be defined, and bone marrow smear appearance was not predictive of the severity of the disease. Lymphocyte subpopulation analysis was available in 74% of the patients. All patients had a normal CD4/CD8 ratio.
Causes of CPN remain unknown. Some authors suggested that CPN associated with neutrophil autoantibodies is a distinct entity.2,10 Wlodarski et al have described the presence of a T-cell clone in some CPN patients reminiscent of neutropenia associated with large granular leukemia.8 To better understand their clinical impact, we compared these subgroups of patients with others CPN patients of our cohort. Only 6 of the 47 patients who were tested had a T-cell clone. There was no association between the presence of a T-cell clone and any clinical or biological feature at diagnosis or with the outcome. Previous studies reported frequencies of neutrophil autoantibodies varying from 15% to 36% and failed to show an impact of their presence on the incidence of infection.4,14 Twenty-three patients (34.8%) in our cohort had neutrophil autoantibodies. Half of the patients with a T-cell clone also showed neutrophil autoantibodies suggesting that these biological features are not mutually exclusive and do not indicate specific mechanisms of CPN pathogenesis. Wlodarski et al previously noted this overlap in the first report of the association between CPN and CD8 clonal T-cell expansions.8 In our study, the specificity of the neutrophil antibody was identified in only 10 patients (43.5%). Detection of granulocyte-specific antibodies is highly variable and depends on studies, contexts, and methods.5,6,21 Regardless of the method used, the low antibody titers and their low avidity for the target antigen make them difficult to detect.22 MAIGA could allow the detection of neutrophil antibodies against few known antigens even when GAT and GIFT fail but is not routinely performed. The sex-ratio imbalance in our study is in keeping with the hypothesized autoimmune mechanism of CPN.23 An immune mechanism is also suggested by the high prevalence of other autoimmune markers in our cohort. Indeed, even though none of our patients had overt autoimmune disease, 47% of them had a biological marker of autoimmunity.
One of the main results of this study is the low rate of patients with severe bacterial infections despite the low ANC and the long follow-up. Some patients present with very severe neutropenia for more than 30 years and remain asymptomatic without any treatment, whereas a few experience severe bacterial infections. No factor apart from the severity of neutropenia at diagnosis was associated with the occurrence of severe infections during follow-up. Our results are in agreement with previously published data in 49 patients with CPN in which the infection rate was 26% and correlated with the severity of neutropenia.14
Twenty-six of the 50 patients who received G-CSF were treated sporadically. The median cumulative days of treatment of these patients was only 111 days, and the indication for receiving G-CSF was not uniform. We therefore excluded these patients from the evaluation of the possible benefit of G-CSF on the prevention of severe bacterial infections. We analyzed only the 24 patients who received G-CSF as part of a planned treatment (median cumulative duration of treatment, 1415 days). Sixteen severe infections developed in this subgroup. The incidence rates of severe bacterial infections were not different before and after the initiation of G-CSF, respectively. The low event counts in this study may explain the absence of statistical significance. Alternatively, although the majority of the patients had hematologic and clinical response to G-CSF, we cannot exclude that permanent G-CSF may not prevent severe bacterial infections in this population. Overall, the majority of the patients in this study received no G-CSF or only sporadically and nevertheless had a favorable outcome. These results are in agreement with previous smaller studies of CPN.24-26 As a curative approach, our results suggest, however, that G-CSF is an appropriate first-line treatment in severe CPN. Indeed, 96% of the patients had clinical and hematologic response to G-CSF with a good tolerance and safety profiles. Other treatments (mainly methylprednisolone, cyclophosphamide, and methotrexate) were effective in less than half of the patients, and relapses were observed at treatment withdrawal in nearly all the patients. Kyle and Linman have previously reported the absence of efficacy of steroid.1 Efficacy of cyclosporine, intravenous immunoglobulin, and rituximab has only been reported occasionally with low response and high relapse rates.24,27-31 Thus, immunosuppressive therapy should only be proposed to symptomatic patients who have failed G-CSF. The extended follow-up in our study allows us to conclude that G-CSF is safe in severe CPN as no myelodysplasic syndrome or acute myeloid leukemia was reported in these prospectively registered patients. Five cases of CPN have been reported to develop secondary acute myeloid leukemia in the literature.32-34 Only 1 had received G-CSF therapy before the onset of leukemia. In 2 of these 5 cases, a family history of hematologic and nonhematologic malignancies was suggestive of a genetic predisposition. The Severe Chronic Neutropenia International Registry, as in our study, did not identify an increased risk of myeloid malignancies in patients with CPN.12
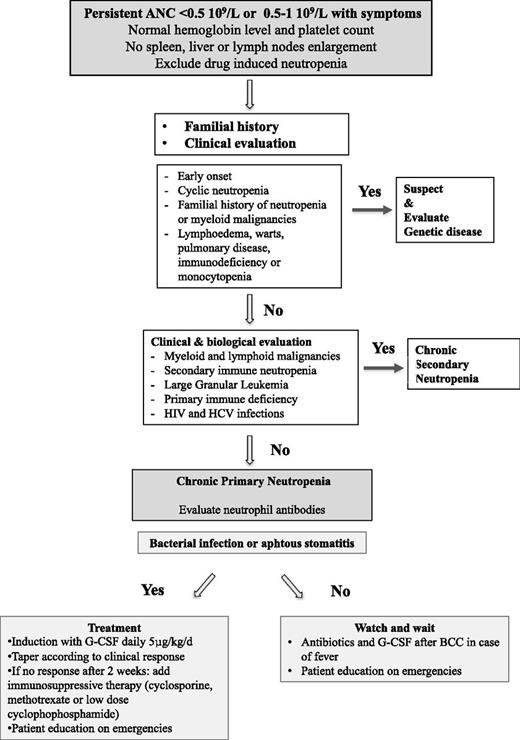
In conclusion, severe adult CPN is a benign entity often diagnosed fortuitously, with a female predominance, sometimes presenting with biological autoimmune features. In the absence of specific biological tests, a complete hematologic and immunologic evaluation is necessary to exclude secondary neutropenia and to ascertain the diagnosis. Screening for gene mutations associated with severe congenital neutropenia is not routinely recommended unless there is a suggestive familial history or a childhood onset. Although severe bacterial infections are rare, they can occur years after the initial diagnosis, and physicians should be cautious and provide advice to severe CPN patients. G-CSF should be administered at the lowest effective dose in case neutropenia persists during or after a severe septic episode. Based on these findings, we propose an approach for diagnosis and treatment of CPN in adults (Figure 3). Further prospective studies associated with centralized hematologic and immunologic analysis at diagnosis will help to increase the understanding of the pathogenesis of severe CPN in adults.
Proposed approach for the diagnosis and treatment of CPN in adults. BCC, blood cell count.
Proposed approach for the diagnosis and treatment of CPN in adults. BCC, blood cell count.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank all the physicians who participated in the study and in the French Severe Chronic Neutropenia Registry.
The French Chronic Neutropenia Registry is supported by grants from Amgen SAS, Chugai SA, Groupements d’Intérêt Scientifique Maladies Rares, RMHE, Institut de Veille Sanitaire, Inserm and the Centre de Reference des Deficits Immunitaires Hereditaires (CEREDIH: the French National Reference Centre for Primary Immune Deficiencies, www.ceredih.fr)
Authorship
Contribution: F.S.d.F., T.L., and J.D. designed the research; F.S.d.F., A.M., B.B., F.S., L.G., G.S., B.V., P.C., M. Michel, C.P., E.O., E.L., L.T., P.M., S.C., N.C., J.M.M., M.G., M. Michallet, L.C., M.A., C.B.-C., J.D., and T.L. provided the patients’ information; F.S.d.F., A.M., F.S., L.C., J.D., and T.L. analyzed the data and wrote the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: J.D. receives grants from Sandoz, and the French Severe Chronic Neutropenia Registry receives financial support from Chugai and Amgen. The remaining authors declare no competing financial interests.
A complete list of the members of the French Severe Chronic Neutropenia Registry appears in “Appendix.”
Correspondence: Flore Sicre de Fontbrune, Hématologie-Greffe, Hôpital Saint-Louis, Paris, France; e-mail: flore.sicre-de-fontbrune@sls.aphp.fr.
Appendix: study group members
The members of the French Severe Chronic Neutropenia Registry are: Dr A. Gourmel, Dr C. Devoldere, Dr V. Li Thiao Te, Dr A. Lutun, Dr X. Rialland, Pr I. Pellier, Dr I. P. Rachieru, Dr J. F. Brasme, Dr P. Bensaid, Pr N. Ifrah, Dr M. Gardembas Pain, Dr S. Francois, Dr F. Boyer Perrard, Dr M. Hunault Berger, Dr A. Schmidt, Dr C. Delorme, Dr F. Bauduer, Dr E. Plouvier, Dr S. Beaussant Cohen, Dr E. Deconinck, Dr G. Daltroff, Dr B. Borm, Dr G. Palenzuela, Dr C. Guitton, Pr C. Goujard, Pr L. De Pontual, Pr Y. Perel, Dr M. Micheau, Dr N. Aladjidi, Dr C. Verite, Pr D. Lacombe, Pr A. Taieb, Pr J. F. Viallard, Dr A. Lemoine, Dr L. Carusu, Dr H. Ansquer, Pr P. Berthou, Dr A. Poirel, Pr P. Cassassus, Dr O. Minckes, Dr D. Bodet, Dr M. Deparis, Pr G. Damaj, Dr O. Reman, Pr. P. Labrune, Pr V. Gadjos, Dr A. Perry, Dr P Trioche, Pr. F. Demeocq, Pr J. Kanold, Dr E. Merlin, Dr E. Dore, Pr C. Borderon, Pr J.o. Bay, Pr O. Tournilhac, Pr D. Bouscary, Pr B. Godeau, Pr M. Michel, Pr J. D. Lelievre, Dr L. Croisille, Dr G. Couillaud, Dr C. Briandet, Pr L. Faivre, Pr C. Thauvin, Dr B. Aral, Dr M. Wetterwald, Dr Y. Hatchuel, Dr J. Gutnecht, Pr J. Y. Cahn, Dr L. Bouillet, Dr J. P. Chouraqui, Dr D. Plantaz, Dr C. Armari, Dr D. Adjaoud, Dr A. Pagnier, Dr Delion, Dr M. Layadi, Dr E. Zairi, Dr Y. Reguerre, Dr M. Jehanne, Dr B. Boumahni, Dr P. Sanyas, Dr P. Morel, Dr B. Dupriez, Dr A. Besancon, Dr D. Martin Coignard, Dr G. Lefevre, Dr B. Catteau, Dr B. Nelken, Dr F. Mazingue, Dr B. Bruno, Dr A. Lambilliote, Dr W. Abouchala, Pr F. Gottrand, Pr J. L. Demory, Pr D. Bordesoules, Dr C. Oudot, Dr E. Piguet, Pr P. Debourdeau, Dr A. Lachaux, Dr L. Le Gall, Dr N. Guffon, Pr. Y. Bertrand, Dr C. Renard, Dr K. Kebaili, Dr L. Nove Josserand, Pr G. Michel, Dr V. Barlogis, Dr I. Thuret, Dr C. Galambrun, Pr H. Chambost, Pr J. Sarles, Dr B. Roquelaure, Dr A. M. Stoppa, Dr A. Charbonnier, Pr G. Kaplanski, Pr N. Schleinitz, Pr J. R. Harle, Dr F. Gouraud, Dr F. Rouquier Thisse, Dr V. Dorvaux, Dr E. Jeziorski, Pr N. Sirvent, Dr S. Haouy, Pr F. Rivier, Pr P. Sarda, Dr L. Pinson, Dr D. Rieu, Pr J. F. Schved, Dr A. Lamour, Dr B. Drenou, Dr M. Benoit, Dr E. Ginglinger, Dr V. Latger, Dr L. Mansuy, Pr P. Chastagner, Dr F. Fouyssac, Pr. J. F. Chabot, Dr D. Ranta, Dr A. Perrot, Pr B. Leheup, Pr F. Feillet, Pr J. P. Bronowicki, Dr B. Romefort, Pr P. Moreau, Dr B Isidor, Dr A. Neel, Dr C. Thomas, Dr F. Rialland, Dr M. Strullu, Dr M. Audrain, Pr. A. Fischer, Pr. S. Blanche, Pr C. Picard, Dr N. Mahlaoui, Dr B. Neven, Dr D. Moshous, Pr F. Rummele, Dr C. Talbotec, Pr O. Goullet, Dr F. Lacaille, Pr. P. De Lonlay, Pr D. Bonnet, Dr M. Rio, Pr E. Macintyre, Pr O. Hermine, Pr. B. Varet, Pr F. Suarez, Dr F. Monpoux, Dr A. Deville, Dr M. Poiree, Dr C. Soler, Pr P. S. Rorlich, Dr. F. Monceaux, Dr S. Perdereau, Dr M. Schoenwald, Dr X. Delbrel, Pr V. Leblond, Dr B. Pellegrino, Dr M. Lachenaud, Dr F. Millot, Dr M. Blayo, Dr O. Fenneteau, Dr K. Yakouben, Pr J. H. Dalle, Dr M. Ouachee, Dr B. Lescoeur, Pr A. Baruchel, Dr B. Brethon, Dr M. L. Bellaiche, Dr S. Gordes Jean, Pr V. Gandemer, Dr S. Bayart, Dr F. Toutain, Dr A. Dabadie, Pr T. Lamy De La Chapelle, Dr F. Dauriac, Dr S. Nimubona, Dr I. Plantier, Pr J. P. Vannier, Pr P. Schneider, Dr A. Marie Cardine, Dr C. Dumesnil, Pr F. Jardin, Pr O. Fain, Pr P. Coppo, Dr L. Garderet, Pr M. Mohty, Dr C. Berger, Pr J.l. Stephan, Dr C. Gay, Pr. E. Oksenhendler, Pr C. Fieschi, Pr J. P. Fermand, Dr L. Galicier, Dr R. Borie, Dr E. Raffoux, Pr H. Dombret, Pr G. Socie, Pr R Peffault De La Tour, Dr F. Sicre De Fontbrune, Pr N. Boissel, Dr E. Lengline, Pr P. Fenaux, Pr. J.p. Lutz, Pr C. Paillard, Pr J.p. Bergerat, Pr R. Herbrecht, Dr F. Maloisel, Dr B. Lioure, Pr J. L. Pasquali, Dr H. Rubie, Dr G. Plat, Dr M. Pasquet, Pr M. Attal, Pr C. Recher, Dr P. Broue, Pr P. Colombat, Dr O. Lejars, Dr P. Blouin, Dr M. Yvert, Dr F. Labarthe, Dr C. Hoarau, Dr G. Dine, Dr C. Manteau, Dr C. Dollfus, Dr J. Donadieu, Pr J. Landman, Pr. G. Leverger, Dr A. Auvrignon, Dr M. D. Tabone, Dr J. C. Petit, Dr S. Fasola, Dr R. Favier, Pr H. Lapillonne, Dr P. Ballerini, Pr P. Tounian, Dr B. Dubern, and Dr B. Cagnard.
References
Author notes
J.D. and T.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal