Key Points
Functional reversion of a germline CARD11 mutation in T cells is associated with the development of Omenn syndrome.
Defective thymic T-cell development and peripheral lymphopenia are no prerequisite for the development of Omenn syndrome.
Abstract
Omenn syndrome (OS) is a severe immunodeficiency associated with erythroderma, lymphoproliferation, elevated IgE, and hyperactive oligoclonal T cells. A restricted T-cell repertoire caused by defective thymic T-cell development and selection, lymphopenia with homeostatic proliferation, and lack of regulatory T cells are considered key factors in OS pathogenesis. We report 2 siblings presenting with cytomegalovirus (CMV) and Pneumocystis jirovecii infections and recurrent sepsis; one developed all clinical features of OS. Both carried homozygous germline mutations in CARD11 (p.Cys150*), impairing NF-κB signaling and IL-2 production. A somatic second-site mutation reverting the stop codon to a missense mutation (p.Cys150Leu) was detected in tissue-infiltrating T cells of the OS patient. Expression of p.Cys150Leu in CARD11-deficient T cells largely reconstituted NF-κB signaling. The reversion likely occurred in a prethymic T-cell precursor, leading to a chimeric T-cell repertoire. We speculate that in our patient the functional advantage of the revertant T cells in the context of persistent CMV infection, combined with lack of regulatory T cells, may have been sufficient to favor OS. This first observation of OS in a patient with a T-cell activation defect suggests that severely defective T-cell development or homeostatic proliferation in a lymphopenic environment are not required for this severe immunopathology.
Introduction
The frequent occurrence of immune-mediated pathology in the context of immunodeficiency is an intriguing paradox. One clinically impressive example is Omenn syndrome (OS).1 Similar to patients with severe combined immunodeficiency (SCID), patients with OS present in early infancy with viral or fungal pneumonia, chronic diarrhea, and failure to thrive. However, unlike SCID, OS is associated with enlarged lymphoid tissues, severe erythroderma, increased IgE levels, and eosinophilia. T-cell counts are normal or elevated with a restricted T-cell receptor repertoire. These highly activated, oligoclonally expanded T cells are autologous and characteristic of Th2 type.2 They have matured in a dysplastic thymus deficient in AIRE expression,3 homeostatically expand in a lymphopenic environment, and are poorly regulated in the periphery.4 Tissue infiltration with these activated T cells dominates this severe immunopathology. Peripheral B cells are typically severely reduced or absent, but plasma cells can be detected in lymphoid organs and are responsible for residual immunoglobulin including excessive IgE production.5,6
Hypomorphic RAG1 or RAG2 mutations were the first genetic cause to be associated with OS,7 but hypomorphic mutations in other genes involved in V(D)J recombination such as DCLRE1C8 and LIG4,9 have also been found. This form of “classical” OS associated with impaired, but not absent, V(D)J recombination accounts for >90% of cases.10 More recently, the clinical features of OS have also been described in patients with mutations in other SCID-causing genes. These patients carried hypomorphic mutations in the ADA, IL2RG, IL7R, AK2, or RMRP genes or had 22q11 deletions.10,11 Some of these “leaky” SCID patients lacked the characteristic B-cell deficiency. To delineate these conditions from “classical OS,” the term Omenn-like syndrome (OLS) has been introduced.10 The common denominator of both conditions, however, is a severe impairment of T-cell development, presumably leading to limited thymic egress of potentially autoreactive T-cell clones.12
The clinical picture of SCID can also be caused by genetic defects allowing normal T-cell development but leading to a severe impairment of T-cell activation.13 These conditions include diseases caused by mutations in ORAI1,14-16 IKBKB,17 or CARD11.18,19 OS has not yet been reported in these disorders, supporting the concept that impaired T-cell development and a priori peripheral T-cell lymphopenia are essential parts of the pathogenesis of the characteristic features of OS.
Here, we describe the first patient with all features of OS associated with a genetic defect of T-cell activation that is associated with impaired development of regulatory, but not of conventional, T cells. We diagnosed CARD11 deficiency in a pair of siblings with a clinical presentation of SCID. One of the patients developed OS associated with a somatic second-site mutation leading to a partial functional reversion in a subset of T cells. This unusual case suggests that OS can occur in the apparent absence of reduced thymic output and lymphopenia-induced homeostatic proliferation.
Material and methods
Informed consent was obtained and the study was approved by the institutional review board (University Medical Center Freiburg, No. 412/09). Animal studies were approved by the Australian National University Animal Experimentation Ethics Committee and the guidelines from the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Histology and immunohistochemistry
Histochemical staining was performed on formalin-fixed, paraffin-embedded tissue specimens using hematoxylin and eosin, Giemsa, and the periodic acid-Schiff reaction. Epitope retrieval was performed in a PT link, pretreatment module (Dako). After blocking nonspecific binding, immunohistochemical staining was conducted using K5005 alkaline phosphatase detection kits in a Dako Plus Autostainer, with primary antibodies listed in supplemental Table 1, available on the Blood Web site. The sections were counterstained with hematoxylin. Sections were evaluated using a Zeiss Imager.M1, and morphometric analysis was calculated as FldAreaP, frame area [μm2] (Carl Zeiss Microscopy, Oberkochen, Germany).
Flow cytometry
Antibodies for flow cytometry are listed in supplemental Table 1. Regulatory T cells were stained using the Human Regulatory T-cell Staining Kit (eBioscience, Affymetrix). Early T-cell activation and cytokine production were analyzed as described.20 For IκB degradation and NF-κB p65 phosphorylation, 5 × 105 peripheral blood mononuclear cells (PBMC) were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 15 minutes at 37°C, fixed (Cytofix, BD Biosciences), and permeabilized (Phosflow Perm III, BD Biosciences), followed by surface and intracellular staining. Data acquisition was performed with a Gallios Flow cytometer (Beckman Coulter). Data were analyzed using FlowJo version 7.2.5 (Tree Star). Bone marrow cells were sorted (purity >95%) on a Moflo device (Beckman Coulter).
T-cell receptor rearrangement
T-cell receptor (TCR)γ chain rearrangements were studied in full-blood DNA according to Biomed-2 protocols.21 Minor modifications were that the forward primers for the variable (V) genes Vγ10, Vγ1-8, Vγ9, and Vγ11 were labeled with different fluorochromes. The polymerase chain reaction (PCR) products were analyzed on an Applied Biosystems 3730XL DNA Analyzer (Life Technologies).
Retroviral reconstitution of PBMC and JPM50.6 Jurkat cells
A pMX based retroviral vector was cloned in a pMX-CARD11-IRES-eGFP configuration22 and transfected into HEK-293 cells together with a pCL-ampho packaging plasmid. Virus-containing supernatant was removed 48 hours later. Primary human PBMC were activated with anti-CD3/anti-CD28 beads (Invitrogen) for 48 hours; seeded on retronectin (Takara)-coated, nontissue, culture-treated 24-well plates (BD Biosciences); and transduced with supernatant-containing retrovirus by spin-infection. IL-2 (100 U/mL, Novartis) and IL-15 (5 ng/mL, Miltenyi Biotec) were added to the cultures every third day. After 10 days, GFP+ and GFP– T cells were sorted and incubated for 24 hours before analysis. CARD11-deficient JPM50.6 Jurkat cells23 were transduced with retroviral supernatant in the presence of Polybrene (4 µg/mL) by centrifugation (800g, 150 minutes, 32°C). After transfection, cells were cultured in RPMI 1640 + 10% fetal calf serum for 2 weeks before fluorescence-activated cell sorting (FACS) of GFP+ cells.
Luciferase reporter assay
Mutant CARD11 alleles were created in the pMX-CARD11-IRES-eGFP construct by site-directed mutagenesis. CARD11-deficient JPM50.6 cells were transfected by electroporation (Neon, Invitrogen) with one of the pMX-CARD11-IRES-eGFP constructs, a firefly luciferase reporter for NF-κB (pBIIX-Firefly24 ) and a cytomegalovirus (CMV) renilla luciferase vector (pRL-CMV Renilla; gift from Dr Schachtrup, CCI Freiburg). One day later, cells were stimulated with PMA (50 ng/mL, Sigma-Aldrich) and ionomycin (1 µg/mL, Sigma-Aldrich) for 5 hours, lysed in passive lysis buffer (Promega), and chemiluminescence was measured upon substrate injection (Dual-Luciferase Reporter Assay System, Promega).
Genomic DNA preparation, RNA isolation, real-time PCR, and quantitative real-time PCR
Genomic DNA was prepared using the QIAmp DNA Blood Kit (Qiagen). RNA was isolated using the RNeasy Mini Kit (QIAGEN) followed by DNAse digestion. 500 ng of total RNA isolated from cells were reverse-transcribed by the SuperScript II Reverse Transcriptase Kit (Life Technologies) with random hexamer priming. Primers and conditions for the amplifications of CARD11 and HPRT cDNAs are available on request.
Genomic and cDNA sequencing
For sequencing, 20 ng genomic DNA or cDNA corresponding to 25 ng total RNA was amplified and sequenced using the Big Dye Terminator v1.1 Cycle Sequencing Kit (Life Technologies). The sequencing products were separated on an Applied Biosystems 3130xl Genetic Analyzer. Primer sequences are available on request.
Immunoblot
1 or 20 µg of total cellular proteins were loaded per lane on 8% or 15% sodium dodecyl sulfate gels. After separation, proteins were blotted semidry on an Immobilon-P membrane (Millipore). Primary antibodies were diluted 1:2000 (rabbit polyclonal anti-CARD11 [full length], Abcam) and 1:1000 (rabbit polyclonal anti-CARD11 [N-terminal], LSBio), respectively. Secondary antibodies were diluted 1:1000 or 1:2000 (goat anti-rabbit IgG [H+L]-horseradish peroxidase conjugate, BioRad). Dilutions were carried out in 10-mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween 20, and 5% milk powder). Blots were developed with the Super Script West Pico Chemiluminescent Substrate Kit (Thermo Scientific).
Retroviral vector cloning, production, and retrovirus-mediated gene transfer into mouse primary B cells
PCR-based site-directed mutagenesis was used to introduce mutations into mouse Card11 cDNA. Amplified PCR products were purified and sequenced on an AB 3730xl DNA Analyzer. Wild-type (WT) and mutant Card11 cDNA were transferred into pMXs-GFP vector. The retroviral constructs were transfected into Phoenix ecotropic packaging cells (ATCC) using calcium phosphate precipitation. The supernatants containing replication-defective retroviral particles were collected and frozen at −80°C until used for transduction. Transfection of cells from HEL-activated B cells from IgHEL transgenic mice with the retroviral vector and subsequent analysis were performed as described previously.25
Results
A novel genetic variant of Omenn syndrome?
The index patient (P1) was a girl born to consanguineous Turkish parents, who presented with congestive heart failure in the context of acute, postnatally acquired CMV infection at 3 months of age. She recovered from multi-organ failure, but failed to eliminate CMV and developed CMV retinitis at 6 months. She was mildly microcephalic and had developmental delay with muscular hypotonia. At age 5 months, she developed progressive eczema evolving into severe generalized erythroderma (Figure 1A), accompanied by generalized lymphadenopathy and hepatosplenomegaly. She had several sepsis episodes caused by Staphylococcus aureus, Enterococcus, and Pseudomonas and died of interstitial pneumonia at 16 months of age. Bronchoalveolar lavage was positive for human metapneumovirus, rhinovirus, and CMV.
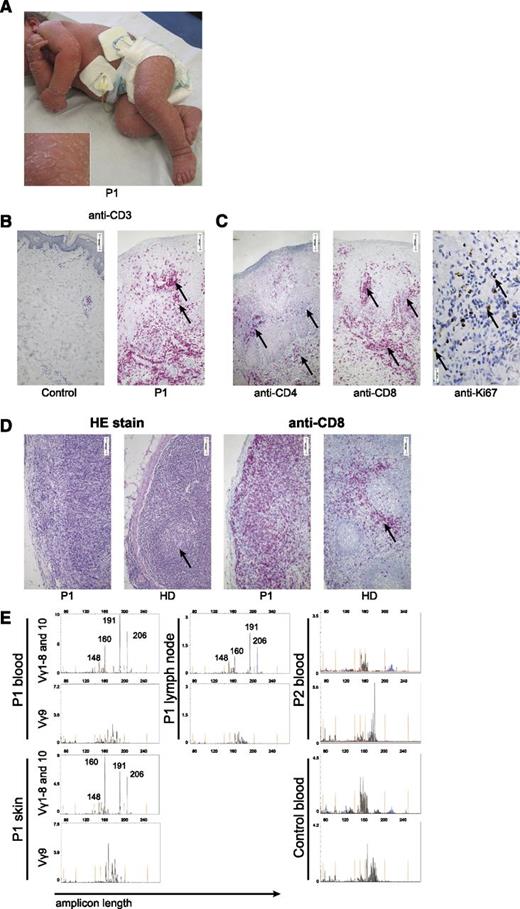
Features of Omenn syndrome in an immunodeficient patient. (A) Desquamative erythroderma in P1 at the age of 9 months. (B-C) Immunohistologic staining of skin biopsies. (B) Anti-CD3 staining in a healthy control and P1. Arrows indicate clusters of infiltrating T cells. Morphometric quantification of 10 high-power fields revealed that CD3 staining covered 17% of the area in the patient compared with 1% in the healthy control. (C) Staining for CD4 and CD8. Ki67 staining reflects high proliferative activity in situ (right). (D) Hematoxylin and eosin (HE) stain (left) and anti-CD8 stain (right) of a lymph node biopsy of P1 and a healthy control. Arrows indicate a normal germinal center in the control (lacking in the patient) and the T-cell zone in the control (diffusely enlarged in the patient). (E) TCRγ rearrangement analysis of samples from blood, skin, and lymph node from P1 and blood of P2 and of a healthy control using primers for Vγ1-8 (upper row, blue), Vγ10 (upper row, black), and Vγ9 (lower row, black). Amplicon length (in bp) is plotted against the fluorescence intensity (arbitrary units). Size standards are indicated in orange.
Features of Omenn syndrome in an immunodeficient patient. (A) Desquamative erythroderma in P1 at the age of 9 months. (B-C) Immunohistologic staining of skin biopsies. (B) Anti-CD3 staining in a healthy control and P1. Arrows indicate clusters of infiltrating T cells. Morphometric quantification of 10 high-power fields revealed that CD3 staining covered 17% of the area in the patient compared with 1% in the healthy control. (C) Staining for CD4 and CD8. Ki67 staining reflects high proliferative activity in situ (right). (D) Hematoxylin and eosin (HE) stain (left) and anti-CD8 stain (right) of a lymph node biopsy of P1 and a healthy control. Arrows indicate a normal germinal center in the control (lacking in the patient) and the T-cell zone in the control (diffusely enlarged in the patient). (E) TCRγ rearrangement analysis of samples from blood, skin, and lymph node from P1 and blood of P2 and of a healthy control using primers for Vγ1-8 (upper row, blue), Vγ10 (upper row, black), and Vγ9 (lower row, black). Amplicon length (in bp) is plotted against the fluorescence intensity (arbitrary units). Size standards are indicated in orange.
A skin biopsy at 9 months showed massive T-cell infiltration (Figure 1B). CD4+ and predominantly CD8+ TCR αβ+ T cells were present in the skin of P1. A highly proliferative activity was reflected by Ki-67 expression (Figure 1C). A lymph node biopsy at 14 months illustrated few hypoplastic germinal centers lacking a mantle zone, whereas the T-cell zone was enlarged as a result of CD8+ T-cell expansion (Figure 1D). Analysis of Vγ rearrangement in T cells revealed at least 4 dominant peaks of identical CDR3 size before an oligoclonal background, which were detected in skin, blood, and lymph nodes (Figure 1E).
At 3 months of age, in the context of disseminated CMV infection, the patient had moderate lymphopenia with normal subset distribution, little evidence of T-cell activation, and a normal fraction of naïve T cells (Table 1).26,27 In the subsequent months, in parallel to the erythroderma, she developed massive T-cell lymphocytosis with dominance of HLA-DR+ CD8+ T cells and a reduced proportion of naïve CD45RA+CD62L+CD4+ T cells (Table 1 and Figure 2A). At this time point, her T-cell receptor variable region (TCRV)-β repertoire displayed oligoclonal T-cell expansions among CD4+ (Figure 2B)28 and CD8+ T cells (supplemental Figure 1). She had eosinophilia, and IgE was elevated (567 U/mL) at the age of 15 months.
Basic immunologic data of P1 and P2 are given with age-matched reference values
| . | P1 . | P2 . | |
|---|---|---|---|
| . | 5 mo . | 14 mo . | 15 mo . |
| CD3+ T cells (cells/µL) | 1095 (2300-6500) | 47 456 (1900-5900) | 8308 (1900-5900) |
| CD4+ (cells/µL) | 597 (1500-5000) | 12 022 (1400-4300) | 2536 (1400-4300) |
| CD8+ (cells/µL) | 498 (500-1600) | 30 372 (500-1700) | 4776 (500-1700) |
| CD19+ B cells (cells/µL) | 26 (600-3000) | 689 (600-2600) | 704 (610-2600) |
| CD16+/CD56+ NK cells (cells/µL) | 49 (100-1300) | 1094 (160-920) | 377 (160-920) |
| CD4+/CD45RA+ (%) | 89 (64-95) | 14 (63-91) | 84 (63-91) |
| CD3+/HLADR+ (%) | 16 (5-8) | 80 (5-8) | 13 (5-8) |
| . | P1 . | P2 . | |
|---|---|---|---|
| . | 5 mo . | 14 mo . | 15 mo . |
| CD3+ T cells (cells/µL) | 1095 (2300-6500) | 47 456 (1900-5900) | 8308 (1900-5900) |
| CD4+ (cells/µL) | 597 (1500-5000) | 12 022 (1400-4300) | 2536 (1400-4300) |
| CD8+ (cells/µL) | 498 (500-1600) | 30 372 (500-1700) | 4776 (500-1700) |
| CD19+ B cells (cells/µL) | 26 (600-3000) | 689 (600-2600) | 704 (610-2600) |
| CD16+/CD56+ NK cells (cells/µL) | 49 (100-1300) | 1094 (160-920) | 377 (160-920) |
| CD4+/CD45RA+ (%) | 89 (64-95) | 14 (63-91) | 84 (63-91) |
| CD3+/HLADR+ (%) | 16 (5-8) | 80 (5-8) | 13 (5-8) |
NK, natural killer cells.
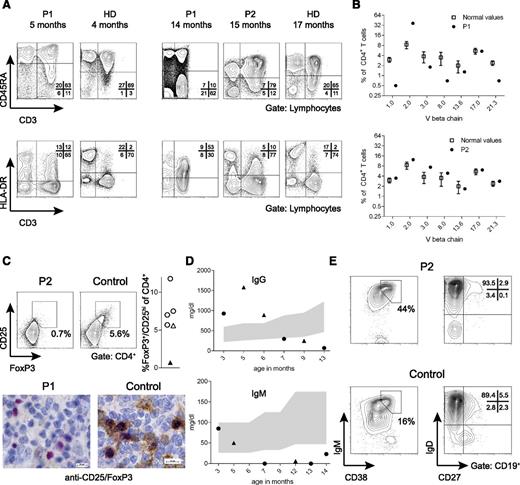
Evolution of basic immunologic parameters over time. (A) CD45RA+ T cells (upper row) and HLA-DR+ T cells (lower row) of P1 at the age of 5 months (uncontrolled CMV infection, beginning eczema) and 14 months (severe erythroderma) compared with T cells from the brother at the age of 15 months (uncontrolled CMV infection, no eczema). Plots from age-matched controls (HD) are shown in comparison. (B) TCRVβ usage among CD4+ T cells as assessed by flow cytometry using TCRVβ-specific antibodies. The percentage of CD4+ T cells expressing the individual TCRVβ chain is shown for P1 and P2 compared with reference values.28 (C) Staining for FoxP3+/CD25+ regulatory T cells in PBMC of P2 and a healthy control. The right panel shows the fraction of regulatory T cells in the day control (open triangle), the patient (black triangle), and 4 age-matched healthy infants (open circles). Below: CD25/FoxP3 stain of a lymph node biopsy from P1 and a control (FoxP3: red; CD25: brown). (D) Serum immunoglobulin levels over time in P1 (circles) and P2 (triangles). The shaded area represents normal values. (E) CD38+IgM+ transitional B cells (left) and IgD–CD27+ switched memory B cells (right) in P2 are shown compared with an age-matched healthy control.
Evolution of basic immunologic parameters over time. (A) CD45RA+ T cells (upper row) and HLA-DR+ T cells (lower row) of P1 at the age of 5 months (uncontrolled CMV infection, beginning eczema) and 14 months (severe erythroderma) compared with T cells from the brother at the age of 15 months (uncontrolled CMV infection, no eczema). Plots from age-matched controls (HD) are shown in comparison. (B) TCRVβ usage among CD4+ T cells as assessed by flow cytometry using TCRVβ-specific antibodies. The percentage of CD4+ T cells expressing the individual TCRVβ chain is shown for P1 and P2 compared with reference values.28 (C) Staining for FoxP3+/CD25+ regulatory T cells in PBMC of P2 and a healthy control. The right panel shows the fraction of regulatory T cells in the day control (open triangle), the patient (black triangle), and 4 age-matched healthy infants (open circles). Below: CD25/FoxP3 stain of a lymph node biopsy from P1 and a control (FoxP3: red; CD25: brown). (D) Serum immunoglobulin levels over time in P1 (circles) and P2 (triangles). The shaded area represents normal values. (E) CD38+IgM+ transitional B cells (left) and IgD–CD27+ switched memory B cells (right) in P2 are shown compared with an age-matched healthy control.
These clinical and immunologic features of OS in a severely immunodeficient patient prompted us to consider SCID variants as the cause of disease. After excluding maternal T cells, we excluded mutations in RAG1, RAG2, Artemis, LIG4, NHEJ1, ADA, IL7R, CHD7, and RMRP. Because of the mild microcephaly, we also tested radiosensitivity and in vitro V(D)J recombination in SV40-transformed fibroblasts, which were normal. Further investigations were then prompted 2 years later, when her younger brother (P2) presented at 6 months of age with Pneumocystis jirovecii pneumonia and persistent CMV infection. He was also mildly microcephalic and had muscular hypotonia, seizures, and developmental delay. He suffered from recurrent sepsis caused by Enterococcus and Klebsiella and died of sepsis at 22 months. There were no skin abnormalities and IgE was normal. At 15 months, in the presence of persistent CMV infection, he had increased CD8+ T-cell counts (Table 1) but a normal T-cell repertoire (Figure 2B), no significant T-cell activation, and a normal fraction of naïve CD4+ T cells (Table 1 and Figure 2A). Of note, CD4+CD25+FoxP3+ regulatory T cells were absent in PBMC from P2, as were FoxP3+/CD25+ cells in a lymph node biopsy of P1 (Figure 2C).
A T-cell and B-cell activation defect associated with impaired NF-κB signaling
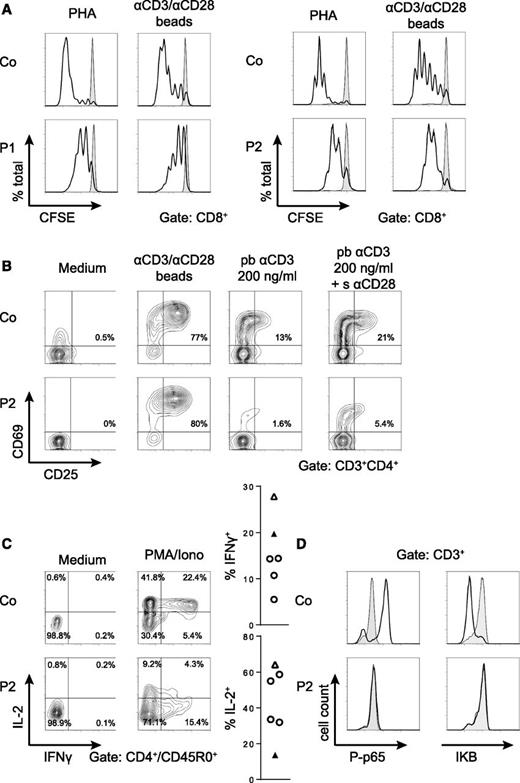
Both children had normal immunoglobulins at 3 and 5 months, respectively. However, by 6 months of age, IgA and IgM were below detection limit and IgG continuously decreased, eventually necessitating immunoglobulin substitution (Figure 2D). Both children produced IgM and IgG to CMV (of low avidity), but did not develop antibodies to diphtheria-tetanus-pertussis or Haemophilus vaccination. Transitional B cells were increased and IgD–CD27+ class-switched memory B cells were reduced in P2 compared with an age-matched healthy donor (Figure 2E). T-cell proliferation was moderately reduced upon phytohemagglutinin (PHA) and anti-CD3/28 stimulation in both patients (Figure 3A), and Ca2+-flux upon OKT3 was normal in P2 (not shown). Upregulation of CD69 and CD25 after PHA activation was normal, but the response to a limited dose of anti-CD3 was poor and showed little increase after addition of anti-CD28 (Figure 3B). Expression of interferon-γ was normal, but expression of IL-2 reduced in T cells of P2 after stimulation with PMA/ionomycin (Figure 3C). Consistent with this pattern of findings, PMA did not induce IκB degradation or NF-κB p65 phosphorylation in P2 T cells (Figure 3D). The material of P1 was not available for these analyses.
A T-cell activation defect affecting the NF-κB pathway. (A) Proliferation of CD8+ T cells from P1 and P2 measured by carboxyfluorescein succinimidyl ester dilution after a 6-day stimulation of PBMC with PHA or anti–CD3/CD28-coated beads (black line). Unstimulated cells are represented by the gray area. Similar results were obtained for CD4+ T cells. Proliferation assays were performed twice with similar results. (B) Early T-cell activation as assessed by CD25/CD69 expression in P2 CD4+ T cells upon overnight stimulation of PBMC with the indicated stimuli. (C) Percentages of IL-2 and IFNγ producing CD45RO+ CD4+ T cells from a day control and P2 measured after a 4-hour stimulation of PBMC with PMA/ionomycin in the presence of Brefeldin A. Graphs represent data from 4 different healthy controls (open circles), the day control (open triangles), and the patient (black triangle). (D) Phosphorylation of p65 (left panel) and degradation of IκB (right panel) in T cells from P2 and a healthy control after stimulation with PMA/ionomycin for 15 minutes (black line) compared with medium control (gray area). All activation assays (B-D) were performed at least 3 times with similar results.
A T-cell activation defect affecting the NF-κB pathway. (A) Proliferation of CD8+ T cells from P1 and P2 measured by carboxyfluorescein succinimidyl ester dilution after a 6-day stimulation of PBMC with PHA or anti–CD3/CD28-coated beads (black line). Unstimulated cells are represented by the gray area. Similar results were obtained for CD4+ T cells. Proliferation assays were performed twice with similar results. (B) Early T-cell activation as assessed by CD25/CD69 expression in P2 CD4+ T cells upon overnight stimulation of PBMC with the indicated stimuli. (C) Percentages of IL-2 and IFNγ producing CD45RO+ CD4+ T cells from a day control and P2 measured after a 4-hour stimulation of PBMC with PMA/ionomycin in the presence of Brefeldin A. Graphs represent data from 4 different healthy controls (open circles), the day control (open triangles), and the patient (black triangle). (D) Phosphorylation of p65 (left panel) and degradation of IκB (right panel) in T cells from P2 and a healthy control after stimulation with PMA/ionomycin for 15 minutes (black line) compared with medium control (gray area). All activation assays (B-D) were performed at least 3 times with similar results.
CARD11 deficiency is the cause of the immunodeficiency
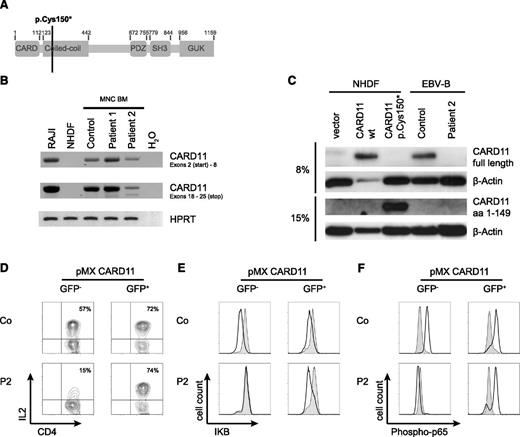
The clinical and immunologic findings in combination with homozygosity mapping prompted us to consider CARD11 deficiency.18,19 Sequencing of the CARD11 gene in fibroblast DNA from P1 and granulocyte DNA from P2 revealed a homozygous C>A transversion in codon 150, leading to a premature stop codon (Figure 4A). RNA analysis revealed transcription of the mutated gene that was reduced in bone marrow mononuclear cells in P2 but not in P1 (Figure 4B). No CARD11 protein was detected by immunoblot analysis in an Epstein-Barr virus line of P2 using different antibodies, allowing recognition of either full-length or truncated CARD11 (Figure 4C). In contrast, the truncated protein was detected in normal human dermal fibroblast (NHDF) cells transduced with the mutant allele (Figure 4C). Because some features of our patients such as initially normal immunoglobulin levels and microcephaly were inconsistent with previous reports on CARD11 deficiency, we reconstituted PBMC from P2 with WT CARD11 by retroviral transduction with a vector encoding a CARD11-IRES-eGFP transgene. Successfully transduced T-cells identified by eGFP expression (8%-12% of culture) showed restored IL-2 production (Figure 4D) and NF-κB signaling (Figure 4E-F), indicating that CARD11 deficiency was the cause for the functional T-cell defect.
A non-sense mutation in the CARD11 gene is the cause of the T-cell activation defect. (A) Model of the CARD11 gene illustrating the site of the mutation in P1 and P2. (B) real-time PCR for the detection of CARD11 RNA isolated from bone marrow mononuclear cells. Amplicons spanning the 5′ and the 3′ ends of CARD11 cDNA. RAJI cells served as a positive control, NHDF as a negative control for CARD11 expression. HPRT cDNA amplification was used to prove cDNA integrity. (C) Immunoblot for detection of CARD11 proteins. Lysates of CARD11-deficient NHDF cells transfected with expression plasmids for WT or the mutant p.Cys150* CARD11 as well as Epstein-Barr virus lymphoblastic cell lines from P2 and a healthy donor were probed for CARD11. The antibodies recognized full-length protein (upper row) or the truncated CARD11 protein (third row). 20 µg protein was loaded for each lane except for NHDF/CARD11 WT (only 1 µg protein loaded). β-Actin was probed as loading control. (D) T cells of P2 and a healthy control were purified, transduced with WT CARD11 by retroviral transfection with pMX-CARD11-IRES-GFP, and tested for expression of IL-2 4 hours after stimulation with PMA/ionomycin. The gates were set on GFP– cells (no transfection) and GFP+ cells (transfection). (E-F) IκB degradation and p65 phosphorylation in retrovirally transduced T cells was measured after stimulation with PMA/ionomycin for 15 minutes (black line). The gray area represents unstimulated cells. All transfection experiments were repeated at least twice with similar results.
A non-sense mutation in the CARD11 gene is the cause of the T-cell activation defect. (A) Model of the CARD11 gene illustrating the site of the mutation in P1 and P2. (B) real-time PCR for the detection of CARD11 RNA isolated from bone marrow mononuclear cells. Amplicons spanning the 5′ and the 3′ ends of CARD11 cDNA. RAJI cells served as a positive control, NHDF as a negative control for CARD11 expression. HPRT cDNA amplification was used to prove cDNA integrity. (C) Immunoblot for detection of CARD11 proteins. Lysates of CARD11-deficient NHDF cells transfected with expression plasmids for WT or the mutant p.Cys150* CARD11 as well as Epstein-Barr virus lymphoblastic cell lines from P2 and a healthy donor were probed for CARD11. The antibodies recognized full-length protein (upper row) or the truncated CARD11 protein (third row). 20 µg protein was loaded for each lane except for NHDF/CARD11 WT (only 1 µg protein loaded). β-Actin was probed as loading control. (D) T cells of P2 and a healthy control were purified, transduced with WT CARD11 by retroviral transfection with pMX-CARD11-IRES-GFP, and tested for expression of IL-2 4 hours after stimulation with PMA/ionomycin. The gates were set on GFP– cells (no transfection) and GFP+ cells (transfection). (E-F) IκB degradation and p65 phosphorylation in retrovirally transduced T cells was measured after stimulation with PMA/ionomycin for 15 minutes (black line). The gray area represents unstimulated cells. All transfection experiments were repeated at least twice with similar results.
Omenn syndrome is associated with a somatic second-site mutation in CARD11
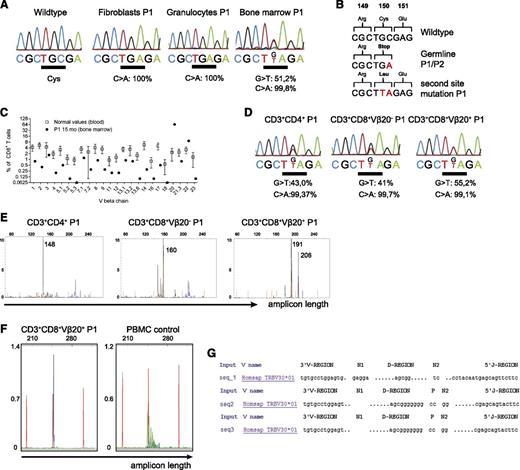
The genetic diagnosis of a T-cell activation defect was difficult to reconcile with development of OS in P1. We considered that an additional genetic event could have favored the oligoclonal T-cell proliferation and therefore studied the expanded T cells in more detail. Because no PBMC were available, bone marrow of P1 was investigated. The bone marrow was highly infiltrated by T cells (94% of all cells), and genetic analysis revealed the germline mutation c.450T>A (Figure 5A). However, in DNA isolated from the bone marrow, but not from peripheral granulocytes, an additional nucleotide exchange c.449G>T was found within the same codon (Figure 5A). This mutation reverses the stop codon (p.Cys150*) and allows continued translation with incorporation of a Leucine residue (p.Cys150Leu). Approximately 50% c.449G>T allele was found in bone marrow, suggesting that most cells in the bone marrow carried the additional somatic mutation on one allele (Figure 5A). The different genetic constellations and their consequences are summarized in Figure 5B.
A somatic second-site mutation in the T-cell compartment is associated with oligoclonal expansion of T cells. (A) Quantitative sequence analysis of the CARD11 gene from the indicated cell populations. Percentages indicate the frequency of the mutated allele (Mutation Surveyor DNA Variant Analysis software). (B) Alterations of the CARD11 sequence and its consequences on protein level are shown. (C) TCRVβ usage among CD8+ T cells in the bone marrow as assessed by flow cytometry using TCRVβ chain-specific antibodies. The percentage of CD8+ T cells expressing the indicated TCRVβ chain is shown for P1 compared with reference values.28 (D) The indicated T-cell populations were purified from bone marrow of P1 by FACS sorting (>95% purity) for quantitative sequence analysis as in (A). (E) TCRγ rearrangement analysis of CD4+, CD8+/Vβ20+, and CD8+/Vβ20– T cells isolated from bone marrow of P1 using primers for Vγ1-8 (blue) and Vγ10 (black). Amplicon length (in bp) is plotted against the fluorescence intensity (arbitrary units). Size standards are indicated in orange. (F) Vβ20-specific primers were used to amplify the Vβ region of FACS-sorted CD8+/Vβ20+ T cells (left) and PBMC from a healthy control (right). (G) PCR products from (F) were sequenced and V(D)J junction sequences were analyzed with IMGT/V-Quest (imgt.org).
A somatic second-site mutation in the T-cell compartment is associated with oligoclonal expansion of T cells. (A) Quantitative sequence analysis of the CARD11 gene from the indicated cell populations. Percentages indicate the frequency of the mutated allele (Mutation Surveyor DNA Variant Analysis software). (B) Alterations of the CARD11 sequence and its consequences on protein level are shown. (C) TCRVβ usage among CD8+ T cells in the bone marrow as assessed by flow cytometry using TCRVβ chain-specific antibodies. The percentage of CD8+ T cells expressing the indicated TCRVβ chain is shown for P1 compared with reference values.28 (D) The indicated T-cell populations were purified from bone marrow of P1 by FACS sorting (>95% purity) for quantitative sequence analysis as in (A). (E) TCRγ rearrangement analysis of CD4+, CD8+/Vβ20+, and CD8+/Vβ20– T cells isolated from bone marrow of P1 using primers for Vγ1-8 (blue) and Vγ10 (black). Amplicon length (in bp) is plotted against the fluorescence intensity (arbitrary units). Size standards are indicated in orange. (F) Vβ20-specific primers were used to amplify the Vβ region of FACS-sorted CD8+/Vβ20+ T cells (left) and PBMC from a healthy control (right). (G) PCR products from (F) were sequenced and V(D)J junction sequences were analyzed with IMGT/V-Quest (imgt.org).
Vβ repertoire analysis revealed a dominant CD8+ TCRVβ20-expressing population, representing ∼70% of total bone marrow CD8+ T cells (Figure 5C). A second, Vβ2-expressing population was detected among CD4+ T cells (not shown; see also Figure 2B). To clarify whether these expansions were related to the second-site mutation, we sorted CD4+, CD8+/Vβ20+, and CD8+/Vβ20– T cells for further genetic analysis. The second-site mutation was most abundant in the CD8+Vβ20+ cells, but also found among CD8+/Vβ20– T cells and CD4+ T cells (Figure 5D). Amplification of the CDR3 region of cDNA from the sorted CD8+/Vβ20– population with Vβ20-specific primers suggested clonality (Figure 5E-F); however, sequencing of the PCR products revealed at least 2 different sequences, indicating oligoclonality (Figure 5G). Also CD8+/Vβ20– T cells and CD4+ T cells from bone marrow contained peaks of the same sizes as in other compartments (Figure 1E), but against an oligoclonal background (Figure 5E). Thus, the second-site mutation could be observed in several expanded CD4+ and CD8+ bone marrow–infiltrating T-cell populations, but not in granulocytes of P1.
CARD11 p.Cys150Leu restores protein expression and function in patient T cells
To study the consequences of the somatic second-site mutation, CARD11-deficient Jurkat cells (JPM50.6)23 were reconstituted with the WT, the germline loss-of-function (p.Cys150*) or the reverted (p.Cys150Leu) allele by retroviral transduction. Transduced GFP+ cells were sorted, expanded, and analyzed. Although p.Cys150*-transduced cells showed no CARD11 expression, CARD11 was re-expressed in p.Cys150Leu-transduced cells at levels comparable to cells transduced with the WT allele (Figure 6A). Upon PMA/ionomycin stimulation, p.Cys150Leu-transduced cells showed partial reconstitution of p65 phosphorylation (Figure 6B). To better quantify the restored NF-κB signaling, transduced JPM50.6 cells were transiently transfected with an NF-κB responsive firefly luciferase reporter construct. p.Cys150Leu-transduced cells showed an increase of luciferase activity in response to PMA/ionomycin compared with cells transduced with the p.Cys150* or the p.Gln945* loss-of-function allele.18 However, luciferase activity remained lower than that in cells transduced with the WT allele (Figure 6C).
Somatic second-site mutation restores CARD11 expression and NF-κB signaling in patient T cells and Jurkat cell line. CARD11-deficient JPM50.6 cells were reconstituted with the indicated alleles of CARD11 by retroviral transduction with pMX-CARD11-IRES-GFP. GFP+ cells were FACS-sorted and expanded. (A) Immunoblot analysis of CARD11 expression compared with expression of CARD11 in Jurkat cell lines and nontransduced JPM50.6 cells. The blot is representative of 2 independent experiments. (B) Phosphorylation of p65 after stimulation with PMA/ionomycin (black line). The shaded area represents unstimulated cells. The assay was repeated 3 times with similar results. (C) Jurkat, JPM50.6, and CARD11-transduced JPM50.6 cells were transiently transfected with a firefly reporter gene construct responsive to NF-κB and a Renilla reporter under control of a CMV promotor for normalization. Bars represent firefly activity normalized to Renilla activity after stimulation with PMA/ionomycin (black bars) and without stimulation (white bars). Pooled data of 3 independent experiments are shown.
Somatic second-site mutation restores CARD11 expression and NF-κB signaling in patient T cells and Jurkat cell line. CARD11-deficient JPM50.6 cells were reconstituted with the indicated alleles of CARD11 by retroviral transduction with pMX-CARD11-IRES-GFP. GFP+ cells were FACS-sorted and expanded. (A) Immunoblot analysis of CARD11 expression compared with expression of CARD11 in Jurkat cell lines and nontransduced JPM50.6 cells. The blot is representative of 2 independent experiments. (B) Phosphorylation of p65 after stimulation with PMA/ionomycin (black line). The shaded area represents unstimulated cells. The assay was repeated 3 times with similar results. (C) Jurkat, JPM50.6, and CARD11-transduced JPM50.6 cells were transiently transfected with a firefly reporter gene construct responsive to NF-κB and a Renilla reporter under control of a CMV promotor for normalization. Bars represent firefly activity normalized to Renilla activity after stimulation with PMA/ionomycin (black bars) and without stimulation (white bars). Pooled data of 3 independent experiments are shown.
The CARD11 p.Cys150Leu variant does not confer gain-of-function activity in murine B cells
Some gain-of-function (GOF) CARD11 alleles have been associated with B-cell lymphoproliferation,29,30 and a GOF effect could in principle explain the lymphoproliferative features of OS in P1. To study the impact of the second-site mutation on the cellular behavior of lymphocytes, we made use of a murine model that has previously been established to evaluate human CARD11 GOF mutations.25 Splenocytes of IgHEL transgenic mice were isolated 6 hours post-stimulation with an acute pulse of HEL antigen and retrovirally transduced with CARD11-IRES-GFP constructs containing WT CARD11, CARD11 p.Cys150Leu, or the GOF allele p.Leu232_Lys233insIle (Figure 7A). After 2 days of culture with anti-CD40, the cells were further maintained without antigen or anti-CD40, and the spontaneous proliferation of GFP+ cells was determined 1 and 2 days later. As expected, a strong proliferative response could be seen in cells transduced with the GOF allele p.Leu232_Lys233insIle, whereas no proliferative response was seen in cells transduced with p.Cys150Leu or the WT allele (Figure 7B-C). Consistently, NF-κB–driven expression of CD25 or CD86 was increased in B cells transduced with p.Leu232_Lys233insIle but was comparably low in B cells transduced with WT or p.Cys150Leu CARD11 alleles (Figure 7D). Finally, spontaneous NF-κB–driven luciferase activity was drastically increased in p.Leu232_Lys233insIle-transduced cells but low in the other 2 cell lines (Figure 7E). These results further support that although p.Cys150Leu restores protein expression and function, it does not possess GOF activity.
The CARD11 p.Cys150Leu variant does not confer GOF properties to murine B cells. (A) Schematic representation of the retroviral vector used for transduction of murine B cells. Mutations are indicated. The experimental procedure is shown on the right. (B) The frequency of transduced cells was determined by flow cytometric analysis of GFP+ signals at day 0, day 1, and day 2. (C) The total number of GFP+ cells was determined at different time points during the culture period, and proliferation was assessed by normalization to the number of GFP+ cells at day 0. (D) Expression of CD25 (left) and CD86 (right) was determined in triplicates at day 1. Gate was set on GFP+ cells. Representative data from 2 independent experiments are shown. (E) NF-κB–induced luciferase activity was normalized to constitutive Renilla luciferase signal. The graph shows technical triplicates. All data shown are representative for data obtained in 2 independent experiments.
The CARD11 p.Cys150Leu variant does not confer GOF properties to murine B cells. (A) Schematic representation of the retroviral vector used for transduction of murine B cells. Mutations are indicated. The experimental procedure is shown on the right. (B) The frequency of transduced cells was determined by flow cytometric analysis of GFP+ signals at day 0, day 1, and day 2. (C) The total number of GFP+ cells was determined at different time points during the culture period, and proliferation was assessed by normalization to the number of GFP+ cells at day 0. (D) Expression of CD25 (left) and CD86 (right) was determined in triplicates at day 1. Gate was set on GFP+ cells. Representative data from 2 independent experiments are shown. (E) NF-κB–induced luciferase activity was normalized to constitutive Renilla luciferase signal. The graph shows technical triplicates. All data shown are representative for data obtained in 2 independent experiments.
Discussion
We describe the first patient with OS in the context of a lymphocyte-activation defect, caused by mutations in the gene encoding CARD11. A somatic second-site mutation leading to partial functional reconstitution in a subset of T cells was associated with the combination of oligoclonal T-cell expansion, severe skin infiltration, lymphoproliferation, and elevated IgE levels characteristic for this disease. Because current evidence indicates that CARD11 deficiency does not cause reduced thymic output and does therefore not result in lymphopenia-induced homeostatic proliferation, our observation suggests that these 2 factors may not be required for the pathogenesis of OS.
OS was recently defined on the basis of 4 major and 10 supportive criteria.31 Apart from reduced T-cell proliferation to antigens (not measured), all 14 criteria were fulfilled in our patient. A key feature of OS is the severe erythroderma caused by dermal infiltration by oligoclonal, highly activated T cells that are not of maternal origin. These T cells can be TCRαβ+ CD4+, CD8+ or double negative,32 or TCRγδ+33 with a highly restricted T-cell repertoire.33,34 In our patient, the skin was predominantly infiltrated by oligoclonal TCRαβ CD8+, but also CD4+ clones carrying identical CDR3 lengths in blood, bone marrow, lymph node, and skin. Such repertoire restriction can already be observed in the thymus of OS patients,35 which is usually dysplastic, lacks cortico-medullary demarcation, and is of poor cellularity.35 Expression of AIRE by medullary thymic epithelial cells is reduced.3 Rag2 mutant mice with features of OS also lack AIRE expression and show profound reduction of thymus-dependent FoxP3+ natural regulatory T cells.36 Based on these observations, current concepts implicate impaired central tolerance, defective generation of regulatory T cells, and homeostatic peripheral proliferation of T cells in a lymphopenic environment in OS pathogenesis.2 However, whether all of these factors are required has been difficult to dissect in humans.
The 3 previously described patients with CARD11 deficiency had normal numbers of T cells, naïve recent thymic emigrants, and TREC levels.18,19,37 Both of our patients initially had a normal fraction of naïve T cells, and P2 had normal to increased T-cell numbers with a normal Vβ repertoire. Moreover, conventional T-cell development, as well as positive and negative selection of thymocytes expressing the MHC class I–restricted HY-TCR transgene, proceeds normally in murine Card11 deficiency.38,39 Although these data indicate normal thymic output in complete CARD11 deficiency, it remains a possibility that in a chimeric situation, revertant T cells have a selective advantage out-competing the deficient T cells, and the thymus may have been seeded with a limited number of revertants with limited specificities. A relevant observation in this context is that reconstitution of irradiated mice with a 4:1 ratio of CARD11−/− and CARD11+/+ bone marrow cells resulted in a 1:1 ratio among peripheral T cells.40 Although this is compatible with a mild disadvantage of CARD11-deficient cells in a competitive situation, all available data indicate that thymic output in CARD11 deficiency is less disturbed compared with classical “leaky” SCID patients who develop OS. Therefore, it is unlikely that reduced thymic output or diversity nor homeostatic proliferation in a lymphopenic environment41 significantly contributed to the development of OS in our patient. In contrast, as shown in mice, CARD11 appears to be essential for thymic development of FoxP3+ natural regulatory T cells.40,42,43 Previously reported CARD11-deficient patients lacked regulatory T cells18,19,37 and we could confirm this in PBMC from P2 and lymph node sections from P1. It is conceivable that absence of regulatory T cells was one factor favoring the occurrence of OS. However, this does not explain why only one of the 2 CARD11-deficient siblings presented with OS.
Eczematous dermatitis has been observed in 1 additional patient with CARD11 deficiency37 and in 2 of 4 patients with mutations in the gene encoding MALT1,44,45 another member of the CARMA1-BCL10-MALT1 (CBM) signalosome complex. T-cell lymphocytic infiltrations have been reported in the skin biopsies of 2 MALT1-deficient patients, but the clonality of the infiltrates was not further investigated. In these patients, IgE was normal and there were no lymphoproliferative manifestations. Late-onset dermatitis has also been reported in unmodulated mice carrying a single amino acid substitution in the coiled-coiled domain of CARD1146 that does not occur in CARD11-null mice.38,39 The skin infiltrates of unmodulated mice showed few T cells with no clonality (A.E., unpublished data, 2012). IgE was elevated, but the mice did not have lymphoproliferation. Thus, it appears that mutations in CBM genes, in particular variants allowing residual signal transmission, predispose to eczematous skin disease. It has previously been proposed that this is caused by a dysbalanced partial inactivation of effector and regulatory T cells.10 However, the lack of the disseminated oligoclonal lymphoproliferation in these mice and patients (including sibling P2) suggests that the predisposition to eczema conferred by some CARD11 mutations was not sufficient to explain an OS phenotype.
We would like to argue that the functional reversion caused by a somatic second-site mutation in the affected CARD11 codon was a relevant factor favoring the evolution of OS in our patient. This second-site mutation could be demonstrated in all T-cell subsets (CD4+, CD8+/Vβ20+, and CD8+/Vβ20–) infiltrating the bone marrow, but not in granulocytes or fibroblasts. This distribution indicates that the reversion occurred before CD4/CD8 lineage commitment, possibly in a prethymic T-cell precursor. The functional reversion resulted in reconstitution of protein expression with partial reconstitution of NF-κB phosphorylation and subsequent transcriptional activity. Mutations in the coiled-coil domain of CARD11 have been associated with congenital B-cell lymphoproliferation29 and B-cell lymphoma.30 However, we documented that the p.Cys150Leu mutation did not elicit increased spontaneous or induced NF-κB signaling, and our murine experiments25 render it unlikely that it conferred autonomous proliferative activity to patient T cells.
Instead, it is likely that the partial reconstitution of CARD11 function conferred an advantage to the revertant T cells relative to the fully CARD11-deficient T cells only in the context of immune triggers, such as virus infections. This idea is strengthened by the observation of a high proportion of naïve T cells in P2 despite active and persistent CMV infection, which can be explained by the T-cell activation defect caused by CARD11 deficiency. The advantage of revertant T cells could have resulted in the poorly controlled proliferative activity in situ reflected by the lymphoproliferation and Ki-67 expression in skin-infiltrating T cells and the dramatic increase in T-cell numbers. The limited fraction of reverted cells with few antigen specificities may have favored the significant skewing of the repertoire. The question of whether the accumulation of the functionally reverted clones in the OS target organs was driven by microbial antigens or autoantigens could not be addressed in this study and is not resolved for OS in general. The persistent CMV infection could represent a relevant chronic stimulus. This would be consistent with the observation that P1 had a relatively normal T-cell compartment at initial presentation (3 months), then developed eczema (5 months), which aggravated to severe erythroderma over the next months associated with the dramatic activation and loss of naïvety in the T-cell compartment (14 months). Hence, we speculate that the reversion provided a proliferative advantage to some virus-specific T-cell clones in the context of a chronic antiviral response, which in the absence of regulatory T cells favored the homing of highly activated cells to OS target organs.
Somatic mosaicism caused by reversion of mutations or second-site mutations restoring protein expression has been described in several primary immunodeficiency diseases,47 including OS associated with RAG148,49 or IL2RG50 mutations. In these 2 conditions, however, the somatic reversion or second-site mutations allowed residual T-cell development in a genetic background, causing severe T-cell lymphopenia and repertoire restrictions. Therefore, impaired thymic development and increased homeostatic proliferation were part of the OS pathogenesis. In contrast, development of OS in our patient was not primarily associated with a low thymic output as a basis of lymphopenia. We rather observed development of OS over time, presumably triggered by the infection with CMV, resulting in hyperactivation of few revertant T-cell clones in a background of a general T-cell activation defect. This first observation of OS in a patient with a T-cell activation defect suggests that an a priori lymphopenic environment is not required for OS pathogenesis.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Andreas Gaa, Tatjana Kersten, Sylvia Kock, Evi Rump, and Ursula Warthorst for excellent technical assistance. They are grateful to Dr Bettina Sehnert and Dr Kristina Schachtrup who provided vectors for the luciferase assays.
This work was supported by the Bundesministerium für Bildung und Forschung (BMBF 01 EO 0803), the Deutschen Forschungsgemeinschaft (DFG SCHW 432/3-1 [K.S., E.H.] and 145/6-1 [S.E.]), and the Jose Carreras Leukämie Stiftung (DJCLS R 10/34) (P.F.).
Authorship
Contribution: S.E. and K.S. initiated the project; S.E., J.R., C.S., T.V., S. Farmand, M.K., B.S., and P.H. took care of the patients; S. Fuchs, U.P., A.R.-E., J.R., A.E., A.S.-G., P.F., Y.J., K.H., and C.G. designed and/or performed experiments and analyzed results; M.R.L. performed genetic analyses; S. Fuchs prepared the figures; and S. Fuchs and S.E. drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Corespondence: Stephan Ehl, Center for Chronic Immunodeficiency, University Medical Center Freiburg, Breisacher Strasse 117, 79106 Freiburg, Germany; e-mail: stephan.ehl@uniklinik-freiburg.de.
References
Author notes
S. Fuchs, A.R.-E., and U.P. contributed equally to this study.
K.S. and S.E. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal