Key Points
Reticulocyte maturation involves the release of intact, inside-out autophagic vesicles with PS exposed on their surface.
Elevated levels of autophagic vesicles on circulating reticulocytes cause PS exposure in patients with SCD.
Abstract
During maturation to an erythrocyte, a reticulocyte must eliminate any residual organelles and reduce its surface area and volume. Here we show this involves a novel process whereby large, intact, inside-out phosphatidylserine (PS)-exposed autophagic vesicles are extruded. Cell surface PS is a well-characterized apoptotic signal initiating phagocytosis. In peripheral blood from patients after splenectomy or in patients with sickle cell disease (SCD), the number of circulating red cells exposing PS on their surface is elevated. We show that in these patients PS is present on the cell surface of red cells in large (∼1.4 µm) discrete areas corresponding to autophagic vesicles. The autophagic vesicles found on reticulocytes are identical to those observed on red cells from splenectomized individuals and patients with SCD. Our data suggest the increased thrombotic risk associated with splenectomy, and patients with hemoglobinopathies is a possible consequence of increased levels of circulating mature reticulocytes expressing inside-out PS-exposed autophagic vesicles because of asplenia.
Introduction
The erythrocyte is one of the most abundant, accessible, and best characterized of human cells but until the recent development of in vitro erythroid culture systems1,2 obtaining large numbers of its precursor cell, the human reticulocyte has been problematic. Reticulocytes are broadly grouped into R1, motile multilobular, and normally confined to bone marrow, and R2, which are nonmotile, much more mechanically stable, and released into peripheral circulation where they comprise ∼2% of red blood cells.3,4 During maturation to an erythrocyte, the reticulocyte must lose ∼20% of its surface area, reduce its volume, and degrade or eliminate residual cytosolic organelles. Current dogma considers that loss of plasma membrane is through the release of endocytosed plasma membrane as exosomes, whereas purging of cellular organelles is executed by autophagy.5
Surface phosphatidylserine (PS) exposure is a well characterized signal for initiating phagocytosis of unwanted cells or cellular material.6 PS is normally located on the intracellular surface of plasma membranes. Relocation to the extracellular surface may occur by activation of a scramblase7 or a bidirectional trafficking process involving cytosolic vesicles.8 PS-exposed red cells are found in the peripheral blood of patients who have undergone splenectomy, or have sickle cell disease (SCD) or thalassemia.9-13
Study design
For details of anonymized patient samples, antibodies used, and full methods, see supplemental Methods, available on the Blood Web site. Briefly, reticulocytes were derived from erythroid cultures and confocal microscopy was performed as described.2 PS was detected using Annexin V fluorescein isothiocyanate (Annexin V-FLUOS Staining Kit [Roche]) as directed by the manufacturer. Mononuclear cells were purified from a waste fraction from a donation of platelets (with informed consent) by apheresis in accordance with the Declaration of Helsinki (and reviewed by the National Health Service National Research Ethics Service).
Results and discussion
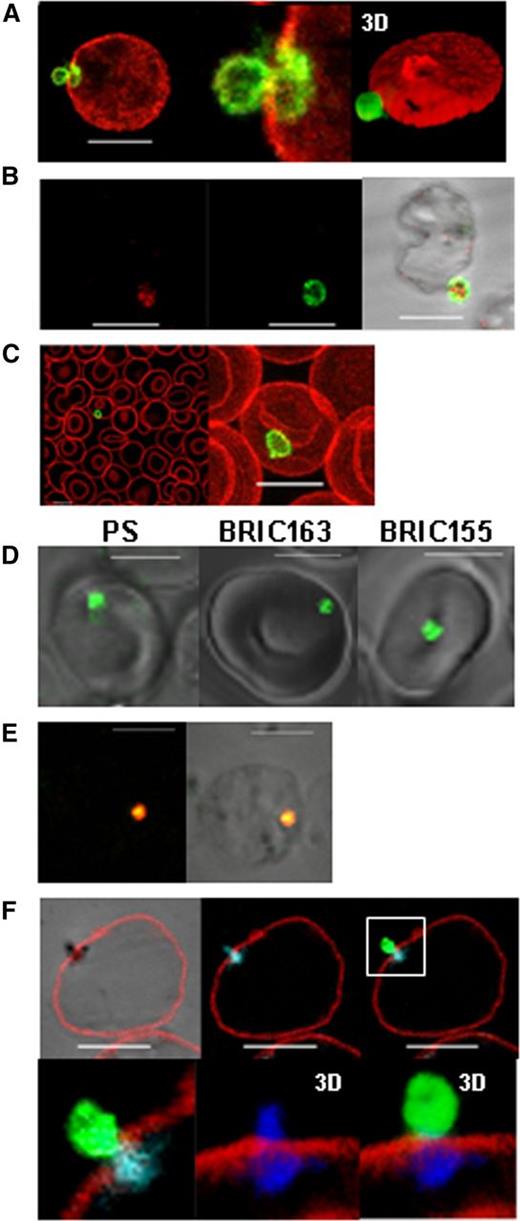
We previously showed2 that maturation of late in vitro-produced reticulocytes involves the generation of glycophorin A (GPA) decorated endocytic vesicles that fuse with autophagosomes to create large autophagic vesicles corresponding to the vacuoles described by Kent et al.14 We used two monoclonal antibodies (mAb) to GPA, which recognize different epitopes on the extracellular domain of the glycoprotein; R10 recognizes a trypsin-sensitive epitope,15 whereas BRIC256 recognizes a trypsin-resistant epitope.16 After trypsin treatment, R10 bound to uncleaved GPA can be distinguished from extracellular proteolytically cleaved GPA in the plasma membrane (Figure 1A). The results show that the endocytosed plasma membrane, which fuses with the autophagosome2 is subsequently expelled from the reticulocyte. Furthermore, the autophagic vesicle appears to be expelled intact. We observe no clear evidence that the vesicle membrane fuses with the plasma membrane as would be expected if residual organelle material were expelled by exocytosis prior to blebbing as previously proposed.2 Dual staining the R10-positive vesicles with the autophagosome marker LC-3 confirmed that the vesicles are identical to those previously described2 (Figure 1B). GPA R10-positive vesicles were observed in a small subset of red cells from the peripheral blood of a normal blood donor (Figure 1C). To ascertain the orientation of these extruding vesicles, cultured reticulocytes were stained for PS and with mAb to intracellular epitopes BRIC16317 (GPA) and BRIC15518 (AE1), and shown to be inside-out (Figure 1D). To control for correct PS staining, we treated red cells with N-ethyl maleimide, followed by Ca2+ and ionomycin as Kuypers et al13 (supplemental Figure 1). We have tested in vitro-produced reticulocytes from numerous different cultures and always observe that 5% to 10% of reticulocytes have an external PS-positive inside-out vesicle. Co-staining for PS and the Golgi marker Giantin (Figure 1E), and BRIC163 and mitochondria (Figure 1F), confirm that these inside-out, PS-exposed vesicles are the same internal autophagic vesicles previously described.2 The Mitotracker-stained emerging vesicle (blue) can be seen straddling the plasma membrane (Figure 1F). We assume that autophagic vesicles expelled by maturing reticulocytes would be phagocytosed in vivo by splenic macrophages in individuals with a functional spleen. Our data suggests that a bidirectional trafficking process, as proposed by Lee et al,8 is used to generate erythrocytes from mature reticulocytes. However, in the maturing human reticulocyte, this apoptotic-like process combines with autophagy to achieve the reduction in surface area and volume, and simultaneously eliminate unwanted residual intracellular organelles in order to form the biconcave erythrocyte.
Release of PS-exposed, inside-out autophagic vesicles is a normal mechanism of reticulocyte maturation. (A) Trypsin-treated in vitro produced reticulocytes were fixed and stained with an anti–trypsin-insensitive GPA antibody (red, BRIC 256) before permeabilization, and then an anti–trypsin-sensitive GPA antibody after permeabilization (green, R10), shown with higher magnification and 3D reconstruction using Volocity software (Perkin Elmer, Waltham, MA). (B) Trypsin-treated in vitro-produced reticulocytes were fixed and stained for trypsin-insensitive GPA (green, R10) and autophagic marker LC3 (red). (C) Trypsin-treated red cells from peripheral blood stained as in (A). Shown in merge and magnification of a maximum projection. (D) Live imaging of in vitro-produced reticulocytes stained for PS, intracellular GPA (BRIC163), and intracellular AE1 (BRIC155) (all green). (E) Fixed and permeabilized in vitro-produced reticulocytes stained for PS (green) and giantin (red). Staining is shown in fluorescence and phase overlay. (F) Fixed unpermeabilized in vitro-produced reticulocytes stained for extracellular AE1 (red, BRAC18) and intracellular GPA (green, BRIC163) followed by Mitotracker (blue) staining post-permeabilization. Shown is a composite of a 2D slice in phase, fluorescence, and overlay images, and 3D reconstructions of the vesicle using Volocity software. All scale bars are 5 µm.
Release of PS-exposed, inside-out autophagic vesicles is a normal mechanism of reticulocyte maturation. (A) Trypsin-treated in vitro produced reticulocytes were fixed and stained with an anti–trypsin-insensitive GPA antibody (red, BRIC 256) before permeabilization, and then an anti–trypsin-sensitive GPA antibody after permeabilization (green, R10), shown with higher magnification and 3D reconstruction using Volocity software (Perkin Elmer, Waltham, MA). (B) Trypsin-treated in vitro-produced reticulocytes were fixed and stained for trypsin-insensitive GPA (green, R10) and autophagic marker LC3 (red). (C) Trypsin-treated red cells from peripheral blood stained as in (A). Shown in merge and magnification of a maximum projection. (D) Live imaging of in vitro-produced reticulocytes stained for PS, intracellular GPA (BRIC163), and intracellular AE1 (BRIC155) (all green). (E) Fixed and permeabilized in vitro-produced reticulocytes stained for PS (green) and giantin (red). Staining is shown in fluorescence and phase overlay. (F) Fixed unpermeabilized in vitro-produced reticulocytes stained for extracellular AE1 (red, BRAC18) and intracellular GPA (green, BRIC163) followed by Mitotracker (blue) staining post-permeabilization. Shown is a composite of a 2D slice in phase, fluorescence, and overlay images, and 3D reconstructions of the vesicle using Volocity software. All scale bars are 5 µm.
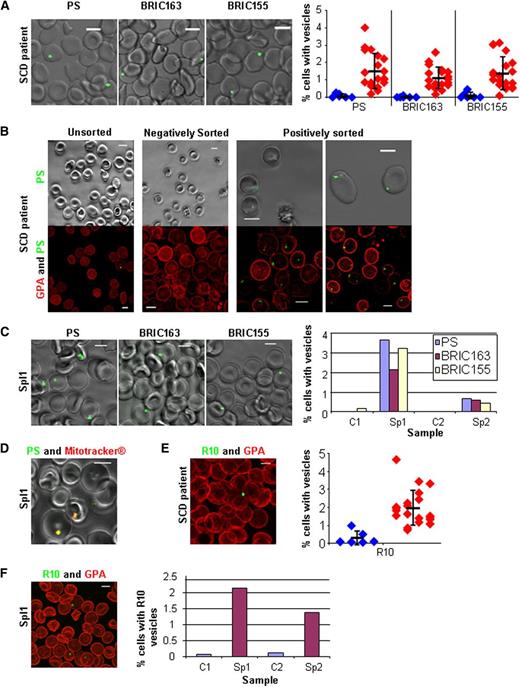
The presence of circulating red cells with exposed PS in patients with hemoglobinopathies is well documented.10,13 The presence of these cells has been linked to a hypercoagulable state through increased thrombin generation and associated platelet activation.12,19 Previous studies demonstrating increased PS expression in SCD were done by flow cytometry and it has been assumed that the PS exposure is uniform over the cell surface. We examined the peripheral blood of SCD patients by live cell imaging. PS was only found in discrete areas of the red cell (Figure 2A) and was not uniformly distributed over the surface, as would be expected if caused by the action of a scramblase.7 Further imaging confirmed the presence of vesicles positive for intracellular GPA and AE1. Quantitation showed all patients had increased numbers of red cells with vesicles positive for PS, and intracellular GPA and AE1 on their surface when compared to controls (Figure 2A). To confirm the PS-positive cells observed by microscopy correlated with those detected by flow cytometry, we recovered PS-positive SCD cells using a cell sorter and imaged. All cells positively sorted for PS showed fluorescence exclusively in large vesicles. Negatively sorted cells did not show any PS fluorescence (Figure 2B). The flow diagrams of the sort are shown in supplemental Figure 2.
PS-exposed autophagic vesicles on red cells from peripheral blood in SCD and after splenectomy. (A) Live imaging of SCD red cells for PS, intracellular GPA (BRIC163), and intracellular AE1 (BRIC155); all green with quantitation from 20 SCD patients (red) and 8 controls (blue) imaging 5 random fields (average n = 439 per field), the thick horizontal line is the mean and standard deviation (SD) from the mean is shown. (B) Live imaging of SCD red cells after sorting for PS (green). Shown in phase overlay (upper panel) and as a 3D reconstruction (lower panel) dual-stained with GPA-546 (BRIC256) (red). (C) Live imaging of splenectomized patient 1 (Spl1, 3 years post-splenectomy) red cells for PS, intracellular GPA (BRIC163), and intracellular AE1 (BRIC155) (all green) with quantitation of Spl1 and splenectomized patient 2 (Spl2, 6 months post-splenectomy) imaging 5 random fields (average n = 375 per field). (D) Live imaging of red cells from Sp1 dual-stained with PS (green) and Mitotracker (red). (E) SCD red cells trypsin treated then fixed, permeabilized, and stained with R10 vesicles (green) and extracellular GPA (red) with quantitation from 20 SCD patients (red) and 8 controls (blue) imaging 5 random fields (average n = 381 per field), the thick horizontal line is the mean and SD from the mean is shown. (F) Spl1 red cells trypsin treated then fixed, permeabilized, and stained with R10 vesicles (green) and extracellular GPA (red) with quantitation from Spl1 and Spl2 with 2 controls imaging 5 random fields (average n = 280 per field). All scale bars are 5 µm.
PS-exposed autophagic vesicles on red cells from peripheral blood in SCD and after splenectomy. (A) Live imaging of SCD red cells for PS, intracellular GPA (BRIC163), and intracellular AE1 (BRIC155); all green with quantitation from 20 SCD patients (red) and 8 controls (blue) imaging 5 random fields (average n = 439 per field), the thick horizontal line is the mean and standard deviation (SD) from the mean is shown. (B) Live imaging of SCD red cells after sorting for PS (green). Shown in phase overlay (upper panel) and as a 3D reconstruction (lower panel) dual-stained with GPA-546 (BRIC256) (red). (C) Live imaging of splenectomized patient 1 (Spl1, 3 years post-splenectomy) red cells for PS, intracellular GPA (BRIC163), and intracellular AE1 (BRIC155) (all green) with quantitation of Spl1 and splenectomized patient 2 (Spl2, 6 months post-splenectomy) imaging 5 random fields (average n = 375 per field). (D) Live imaging of red cells from Sp1 dual-stained with PS (green) and Mitotracker (red). (E) SCD red cells trypsin treated then fixed, permeabilized, and stained with R10 vesicles (green) and extracellular GPA (red) with quantitation from 20 SCD patients (red) and 8 controls (blue) imaging 5 random fields (average n = 381 per field), the thick horizontal line is the mean and SD from the mean is shown. (F) Spl1 red cells trypsin treated then fixed, permeabilized, and stained with R10 vesicles (green) and extracellular GPA (red) with quantitation from Spl1 and Spl2 with 2 controls imaging 5 random fields (average n = 280 per field). All scale bars are 5 µm.
The number of PS-exposed red cells in patients with SCD is higher in those patients who have had a splenectomy.10,11 Hyposplenism is present in the majority of SCD patients before 12 months of age.20 An increased number of PS-exposed red cells has also been observed by flow cytometry in circulating red cells after splenectomy.21 We analyzed red cells from two splenectomized but otherwise healthy individuals. In both cases, splenectomy was performed because of immune-mediated thrombocytopenia and the patients’ hematologic parameters were as expected post-splenectomy. PS at the surface of patient red cells was exclusively on extruding vesicles and not elsewhere on the plasma membrane (Figure 2C). Further imaging confirmed the presence of vesicles positive for intracellular GPA and AE1, and both splenectomized individuals had more PS and intracellular GPA and AE1-positive vesicles on their red cells than controls (Figure 2C). Live dual-staining for PS and mitochondria in SCD red cells showed complete co-localization in these vesicles (Figure 2D). They are analogous to the internal PS-positive vesicles identified in cultured reticulocytes (Figure 1F). Red cells from SCD and splenectomized patients were trypsin-treated and stained with R10, and also found to have elevated levels of internal GPA-decorated vesicles (Figure 2E-F). We provide evidence indicating that PS-exposed red cells described in SCD and splenectomized individuals9-12 are circulating reticulocytes, which cannot be properly processed because the patients lack a functional spleen.
In mature R2 human reticulocytes, we show that the trafficked vesicles have an inside-out orientation and in vivo these vesicles are likely removed from the maturing reticulocyte by the spleen. Failure to remove these vesicles could explain the observed increase in thrombotic events that are reported to occur in splenectomized individuals. If not removed by the spleen, autophagic PS-exposed vesicles are possibly released directly into plasma where they could be as pathologically active as those attached to red cells. Hyposplenism is a feature of SCD19 and failure to remove the vesicles from circulating red cells may exacerbate the clinical severity of disease at sites of vaso-occlusion. Transfusion therapy reduces the percentage of PS-positive red cells circulating in SCD patients, although the mechanism is not clear.10 The development of procedures for removing PS-positive vesicles from circulating red cells ex vivo could provide a useful additional therapy for patients with SCD and other hemoglobinopathies, particularly those patients for whom obtaining sufficient compatible blood for transfusion is problematic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Sabine Taylor (Bristol Institute for Transfusion Sciences, United Kingdom) for assistance in culturing reticulocytes and Dr Andrew Herman (University of Bristol, United Kingdom) for assistance with fluorescence-activated cell sorting.
This work was supported by grants from the Department of Health (England), the National Institute for Health Research, and the Wellcome Trust.
Authorship
Contribution: T.J.M. and R.E.G. designed and performed experiments, analyzed data, and wrote the paper; J.F.F. and N.M.C. performed confocal experiments on erythrocytes; S.T. and E.J.M. provided patients samples and reviewed the paper; and D.J.A. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David J. Anstee, Bristol Institute for Transfusion Sciences, National Health Service Blood and Transplant, Northway, Filton, Bristol BS34 7QH, United Kingdom; e-mail: david.anstee@nhsbt.nhs.uk.
References
Author notes
T.J.M. and R.E.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal