Key Points
Saliva induces bactericidal and DNase resistant NETs in the oral cavity via sialyl LewisX- L-selectin signaling.
Disordered homeostasis in the oral cavity may lead to deficient saliva-mediated NETosis.
Abstract
Neutrophils are essential for host defense at the oral mucosa and neutropenia or functional neutrophil defects lead to disordered oral homeostasis. We found that neutrophils from the oral mucosa harvested from morning saliva had released neutrophil extracellular traps (undergone NETosis) in vivo. The NETosis was mediated through intracellular signals elicited by binding of sialyl LewisX present on salival mucins to l-selectin on neutrophils. This led to rapid loss of nuclear membrane and intracellular release of granule proteins with subsequent neutrophil extracellular trap (NET) release independent of elastase and reduced NAD phosphate-oxidase activation. The saliva-induced NETs were more DNase-resistant and had higher capacity to bind and kill bacteria than NETs induced by bacteria or by phorbol-myristate acetate. Furthermore, saliva/sialyl LewisX mediated signaling enhanced intracellular killing of bacteria by neutrophils. Saliva from patients with aphthous ulcers and Behçet disease prone to oral ulcers failed to induce NETosis, but for different reasons it demonstrated that disordered homeostasis in the oral cavity may result in deficient saliva-mediated NETosis.
Introduction
Neutrophils are pivotal for host defense.1,2 Neutrophils are continuously recruited to the oral mucosae3 and are important for immune defense and tissue homeostasis in the oral cavity, as illustrated by neutropenia and functional neutrophil deficiencies that are associated with gingivitis, periodontitis, and ulcerations.4,5 Indeed, oral ulcers are a characteristic feature of the periods of neutrophil nadirs in cyclic neutropenia.6 In addition to phagocytosis and release of antimicrobial substances, neutrophils can trap and kill bacteria by extracellular extrusion of nuclear DNA as neutrophil extracellular traps (NETs).7 Neutrophils form NETs by 2 distinct mechanisms.8 One mechanism is dependent on the reduced NAD phosphate oxidase activation, elastase, and myeloperoxidase (MPO) activity and can be elicited by phorbol-myristate acetate (PMA), Candida albicans, or uric acid crystals,7,9-11 whereas bacteria and bacterial products can induce NETs by another mechanism involving integrins and Toll-like receptor (TLRs).8,12-14 Apart from host defense,15 NETs have been implicated in diseases like thrombosis, autoimmunity, and gout.11,16,17
To study the fate of neutrophils at a mucosal surface, we harvested neutrophils from the oral mucosa in morning saliva and found that oral neutrophils undergo saliva-induced NETosis in vivo mediated by a novel mechanism elicited by sialyl LewisX-l-selectin signaling resulting in bactericidal and DNase-resistant NETs.
Materials and methods
Detailed information about all materials, methods, and patients are found in the supplementary Materials and Methods available on the Blood web site.
NET induction and immunofluorescence microscopy
Polymorphonuclear leukocytes adhered to coverslips for 15 minutes at room temperature and 15 minutes at 37°C. Neutrophils in RPMI 1640 with 2 mg/mL human serum albumin (HSA) were used as nonstimulated controls. NETs were induced by saliva (undiluted or diluted 1:1 in protein-free saliva), 20 nM PMA, 100 mU/mL glucose oxidase or Staphylococcus aureus (multiplicity of infection [MOI] 30). Cells were fixed, permeabilized, and probed with antibodies against neutrophil elastase18 or myeloperoxidase (DAKO A0398). Secondary antibodies with Alexa488 and Alexa594 (Lifetech) were added and samples were mounted in ProLong Gold anti-fade reagent with 4,6 diamidino-2-phenylindole (DAPI; Lifetech).
NET induction of LAD1 neutrophils
Leukocyte adhesion deficiency type 1 (LAD1) neutrophils were stimulated in solution, cytocentrifuged, and processed for immunohistochemistry similar to neutrophils on coverslips.
Quantification of NETs
Fixed cells were labeled for elastase and stained with DAPI. The elastase positive area in nonstimulated cells with normal polymorphonuclear morphology was quantitated using the algorithm developed by Brinkman et al.19 By using an increase in elastase-positive area of 33% as a cutoff for NET formation we always found colocalization of elastase and DNA in cells defined to have undergone NETosis. Image analysis was performed with public domain software (Fiji).
Bacterial culture
S. aureus (clinical isolate strain 050701) and Streptococcus pyogenes (AP1) were plated for 6 to 8 hours, and overnight cultures were started from plated bacteria. Bacteria from overnight cultures were harvested and cultured for 3 hours (3 hours day culture), washed, and used. The S. aureus (MOI 1:30) from either overnight culture or 3 hours day culture was used for generating NETs. Oral bacteria were harvested from morning saliva from donors and were plated on THY plates and processed similar to S. aureus and S. pyogenes.
Preparation of salival neutrophils from morning saliva
Saliva from healthy donors (ie, without any known systemic or oral disease) was collected into ice-chilled tubes early in the morning before brushing teeth. Samples were incubated with fixative, diluted, cytocentrifuged, and immunostained.
Collection and preparation of saliva and protein-free saliva
Saliva from healthy donors was collected in the morning after teeth brushing and before additional intake of food. The samples were centrifuged and sterile filtered. Further centrifugation through a 3 kDa filter (Millipore) yielded protein-free filtrate (protein-free saliva).
Saliva samples from Behçet disease, systemic lupus erythematosus and recurrent aphthous ulcers were centrifuged and supernatant diluted 1:1 in healthy protein-free saliva was used. Saliva samples from normal donors used in parallel were processed similarly.
Deglycosylation of saliva and mucin and collection salival and mucin glycans
Saliva and mucins were incubated with or without PNGase-F to remove N-linked glycans. Retentates with deglycosylated saliva and mucins were resuspended to their original volume in saliva buffer. Liberated glycans were lyophilized and resuspended to original volume in RPMI 1640.
Inhibition experiments
There were 100 μg/mL anti-sialyl LewisX-antibodies (KM93, Millipore) incubated with salival glycans before neutrophil stimulation. For l-selectin blocking, neutrophils were allowed to adhere, and 50 μg/mL l-selectin blocking antibodies (RnDSystems) were incubated with neutrophils for 15 minutes at 37°C before stimulation.
Neutrophils were coincubated with 10 μM MAPK and mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) inhibitors UO126 (Tocris Biosciences) and SB202190 (Tocris Biosciences) both during adherence and stimulation with saliva.
Intracellular bacterial killing assay
S. pyogenes opsonized with saliva or 20% plasma were resuspended in RPMI 1640 and added to neutrophils (MOI 1:10). Gentamycin was added after 15 minutes during the neutrophil-bacteria coincubation to eliminate extracellular bacteria. The neutrophils were washed and resuspended in RPMI 1640 with HSA (control), RPMI 1640 with HSA and 50 μg/mL sialyl LewisX or saliva. DNase (1 U/mL) was added and cells were lysed and plated for culture.
Bacterial viability assay for NET-bound bacteria
S. aureus (clinical isolate strain 0507101) were added (MOI 1:30) to NETs pregenerated with saliva or PMA. NETs were incubated with bacteria and live/dead staining was performed using the BacLight viability kit (Life Technologies) and then mounted in ProLong Gold Antifade Reagent with DAPI (Life Technologies).
NET degradation assay
Neutrophils in ibiTreat chamber slides (ibidi) were exposed to saliva, S. aureus and PMA to induce NETs, stained with SYTOX orange (Life Technologies), and photographed. Next, bacteria-free medium from an overnight culture of S. pyogenes (AP-1) or fresh saliva was added. Percentage of NET integrity was calculated from ratio of area of staining after addition of saliva or AP-1 supernatant compared with area of staining prior to addition of NET degrading substances.
Ethics statement
Neutrophils and saliva were collected from healthy donors and patients giving informed consent in accordance with the Declaration of Helsinki. The study was approved by the ethics committees of Lund University (2013/728) and the Capital Region of Denmark (H-1-2011-165).
Results
NETs are present in the oral cavity and induced by saliva
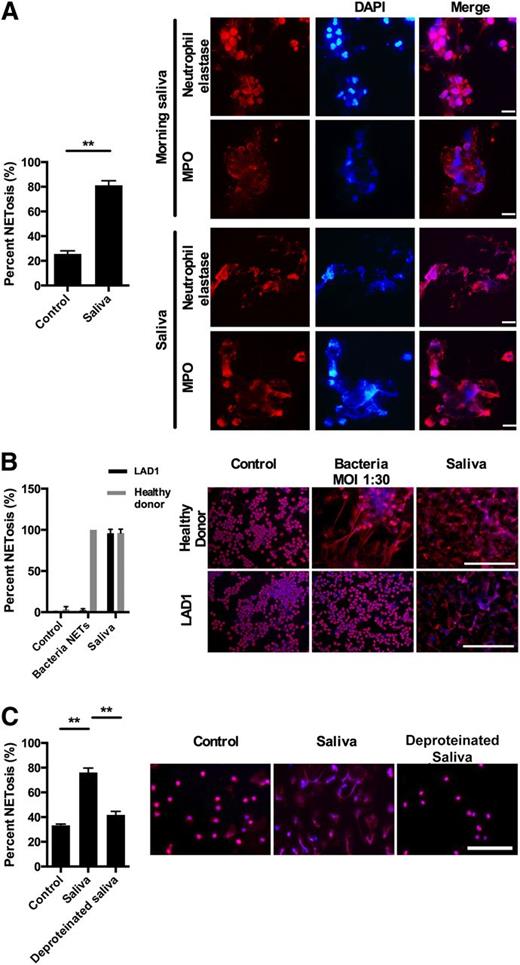
Numerous neutrophils present in morning saliva have undergone NETosis as evidenced by strands of extracellular DNA associated with elastase and MPO, a hallmark of NETosis (Figure 1A). The majority of neutrophils isolated from peripheral blood undergo NETosis within 1 hour incubation with saliva demonstrating that the mere exposure of neutrophils to saliva is sufficient to induce NETosis (Figure 1B). See supplemental Appendix A for detailed statistical analysis of all data presented in the figures.
Saliva induces NETs in the oral cavity. Immunofluorescence microscopy was performed with staining for DNA (DAPI, blue) and for elastase and MPO (both red). All images were acquired using a Nikon Eclipse TE200 equipped with a Hamamatsu C4742-95 CCD camera, using Plan Apochromat 20× and 100× objectives and NIS-elements 3.1 (Nikon) software was used for image acquisition and processing. All images for all figures were acquired and formatted in the same fashion unless stated otherwise. (A) Immunofluorescence microscopy of neutrophils collected from morning saliva demonstrated the presence of NETs judged from presence of extracellular DNA with bound elastase and MPO. Stimulation of neutrophils with saliva caused similar NET formation. The presence of NETS in morning saliva were investigated in 6 donors (5 nonsmokers and 1 smoker) with no systemic disease or known oral or dental disease. NETs were found in all donors with no apparent difference between the smoker and the nonsmokers. Scale bars represent 10 μm. (B) Neutrophils from a 13-month-old patient with LAD1 and a healthy control were stimulated for 1 hour with either saliva or S. aureus or left nonstimulated in RPMI 1640 with HSA. Scale bars denote 200 μm and the error bars denote the difference between 20 randomly selected images. Due to the lack of integrins the neutrophils in this experiment are not stimulated on coverslips but in solution. This gives a lower amount of NETosis in the nonstimulated neutrophils compared with the other experiments in which the neutrophils adhere to coverslips before stimulation. (C) Neutrophils were stimulated with saliva or protein-free saliva. Results from 3 independent experiments are shown (in A and C). Detailed statistical analyses for all figures are included in supplementary Appendix A. Scale bars, 100 μm. **P < .01, columns denote average values and error bars denote standard deviations.
Saliva induces NETs in the oral cavity. Immunofluorescence microscopy was performed with staining for DNA (DAPI, blue) and for elastase and MPO (both red). All images were acquired using a Nikon Eclipse TE200 equipped with a Hamamatsu C4742-95 CCD camera, using Plan Apochromat 20× and 100× objectives and NIS-elements 3.1 (Nikon) software was used for image acquisition and processing. All images for all figures were acquired and formatted in the same fashion unless stated otherwise. (A) Immunofluorescence microscopy of neutrophils collected from morning saliva demonstrated the presence of NETs judged from presence of extracellular DNA with bound elastase and MPO. Stimulation of neutrophils with saliva caused similar NET formation. The presence of NETS in morning saliva were investigated in 6 donors (5 nonsmokers and 1 smoker) with no systemic disease or known oral or dental disease. NETs were found in all donors with no apparent difference between the smoker and the nonsmokers. Scale bars represent 10 μm. (B) Neutrophils from a 13-month-old patient with LAD1 and a healthy control were stimulated for 1 hour with either saliva or S. aureus or left nonstimulated in RPMI 1640 with HSA. Scale bars denote 200 μm and the error bars denote the difference between 20 randomly selected images. Due to the lack of integrins the neutrophils in this experiment are not stimulated on coverslips but in solution. This gives a lower amount of NETosis in the nonstimulated neutrophils compared with the other experiments in which the neutrophils adhere to coverslips before stimulation. (C) Neutrophils were stimulated with saliva or protein-free saliva. Results from 3 independent experiments are shown (in A and C). Detailed statistical analyses for all figures are included in supplementary Appendix A. Scale bars, 100 μm. **P < .01, columns denote average values and error bars denote standard deviations.
Saliva contains numerous microorganisms, and microorganisms are potent inducers of NETosis by a mechanism dependent on β2-integrins.8,12,14 Therefore, we examined whether NETosis induced by saliva is dependent on neutrophil β2-integrins. Neutrophils from a patient with LAD1 lacking β2-integrins formed NETs in response to saliva and PMA stimulation, but failed to form NETs in response to bacteria, demonstrating that saliva-induced NETosis is β2-integrin independent (Figure 1B; supplementary Figure 1).
Incubation of neutrophils with protein-free saliva failed to induce NETosis (Figure 1C) demonstrating that a specific component present in saliva, not merely the hypotonicity of saliva, induces NETosis.
Salival mucins are responsible saliva-induced NETosis through sialyl LewisX-l-selectin-mediated signaling
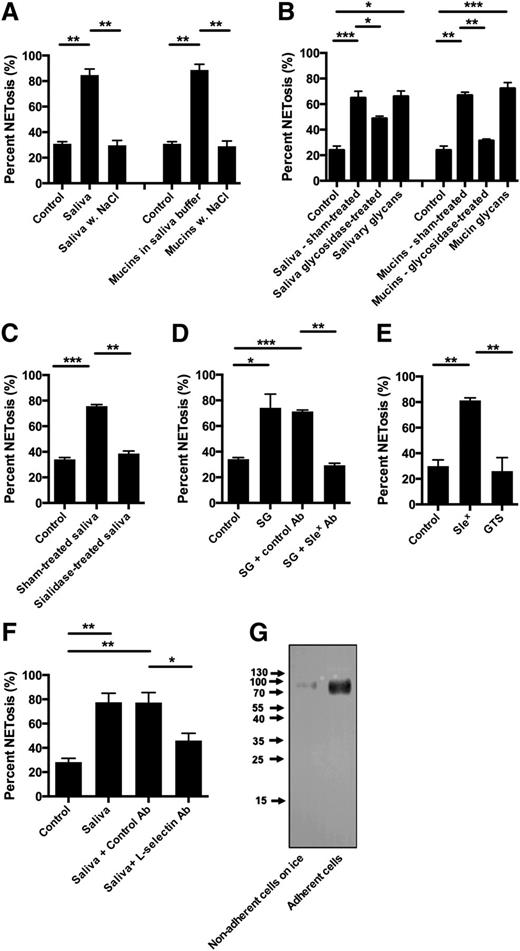
Raising the NaCl concentration in saliva to 140 mM abolished saliva-induced NETosis (Figure 2A). Because the structure of mucins depends on the ionic strength of the medium,20 we tested whether salival mucins induced NETosis. Salival mucins induced NETs when dissolved in buffer with similar ionic concentration as saliva (saliva buffer), but not in medium with 140 mM NaCl (Figure 2A).
Salival glycans induce NETosis mediated by sialyl LewisX – l-selectin-mediated signaling. Neutrophils were stimulated to NETosis and examined by immunofluorescence microscopy. NET formation was quantified as described in “Materials and methods.” Quantification data are shown from 3 independent experiments, except in the experiments with l-selectin blocking antibodies in which the results represent 4 independent experiments. Representative images are shown in supplementary Figure 2. (A) Neutrophils were stimulated both with saliva and salival mucins in saliva buffer with the ionic composition of saliva or in saliva buffer with adjustment of the NaCl concentration to 140 mM. (B) Saliva and mucins were treated with PGNase F to remove N-linked glycancs. Neutrophils were subsequently stimulated with the PGNase F-treated saliva and mucin after removal of the cleaved-off glycans and with these glycans. Lectin blot for N-linked glycans (supplementary Figure 2) demonstrated that not all N-linked glycans were removed by PGNase F treatment. (C) Before stimulation of neutrophils, saliva was treated with sialidase (neuraminidase). (D) Neutrophils were stimulated with isolated salivary glycans (SG) (removed from by PGNase F treatment) in the presence of sialyl LewisX blocking IgM (KM93) or negative control IgM. The glycans were resuspended in RPMI 1640 before incubation with antibodies. (E) Neutrophils were stimulated with either sialyl LewisX tetrasaccharide (SleX) or glucose tetrasaccharide (GTS). Scale bars, 100 μm. (F) Neutrophils were incubated with blocking antibodies to l-selectin antibodies or control antibodies prior to stimulation with saliva. (G) Western blot of l-selectin in the medium of neutrophils adhering to coverslips or incubated on ice. Columns denote average values and error bars standard deviations. *P < .05; **P < .01; ***P < .01, refer to nominal significance of induction or inhibition of NET formation.
Salival glycans induce NETosis mediated by sialyl LewisX – l-selectin-mediated signaling. Neutrophils were stimulated to NETosis and examined by immunofluorescence microscopy. NET formation was quantified as described in “Materials and methods.” Quantification data are shown from 3 independent experiments, except in the experiments with l-selectin blocking antibodies in which the results represent 4 independent experiments. Representative images are shown in supplementary Figure 2. (A) Neutrophils were stimulated both with saliva and salival mucins in saliva buffer with the ionic composition of saliva or in saliva buffer with adjustment of the NaCl concentration to 140 mM. (B) Saliva and mucins were treated with PGNase F to remove N-linked glycancs. Neutrophils were subsequently stimulated with the PGNase F-treated saliva and mucin after removal of the cleaved-off glycans and with these glycans. Lectin blot for N-linked glycans (supplementary Figure 2) demonstrated that not all N-linked glycans were removed by PGNase F treatment. (C) Before stimulation of neutrophils, saliva was treated with sialidase (neuraminidase). (D) Neutrophils were stimulated with isolated salivary glycans (SG) (removed from by PGNase F treatment) in the presence of sialyl LewisX blocking IgM (KM93) or negative control IgM. The glycans were resuspended in RPMI 1640 before incubation with antibodies. (E) Neutrophils were stimulated with either sialyl LewisX tetrasaccharide (SleX) or glucose tetrasaccharide (GTS). Scale bars, 100 μm. (F) Neutrophils were incubated with blocking antibodies to l-selectin antibodies or control antibodies prior to stimulation with saliva. (G) Western blot of l-selectin in the medium of neutrophils adhering to coverslips or incubated on ice. Columns denote average values and error bars standard deviations. *P < .05; **P < .01; ***P < .01, refer to nominal significance of induction or inhibition of NET formation.
To investigate the role of the glycan moieties in mucins, N-linked glycans from saliva and isolated salival mucins were removed by peptide-N-glycosidase F (PNGase F) treatment. This reduced the capacity to induce NETosis, both from saliva and from purified mucins (Figure 2B). The effect of de-glycosylation was most prominent on purified mucins. A lectin blot to detect N-linked glycans (supplementary Figure 2) demonstrated only partial removal of N-linked glycans by PNGase F-treatment of saliva, probably because PNGase F works best on denatured proteins.21 Purified glycans released by PNGase F treatment of saliva and mucins induced NETosis, even in isotonic medium (Figure 2B), clearly indicating that glycans present on mucins are the NET inducing component of saliva, but that these glycans only induce NETosis when mucins maintain the structure imposed by the ionic conditions in saliva.
Salival mucins contain the sialic acid-containing tetrasaccharide sialyl LewisX, which is a ligand for l-selectin22,23 present on neutrophils. Removal of sialic acid by sialidase treatment inhibited the NET-inducing activity of saliva (Figure 2C) demonstrating that the NET-inducing activity is due to a sialic acid-containing saccharide. Preincubation of saliva glycans with sialyl LewisX antibodies before adding to neutrophils abrogated the NET-inducing activity (Figure 2D). Purified sialyl LewisX tetrasaccharide induced NETosis, whereas glucose tetrasaccharide did not (Figure 2E), thus proving that sialyl LewisX present in salival mucins is the moiety present in saliva that induces NETosis. Preincubation of neutrophils with blocking anti l-selectin antibodies prior to incubation with saliva inhibited saliva-induced NETosis (Figure 2F), demonstrating that NETosis is induced by sialyl LewisX binding to l-selectin on neutrophils.
l-selectin is shed when neutrophils adhere to endothelial cells,24 but only partially.25 Western blot of medium from neutrophils adherent to coverslips demonstrated shedding of l-selectin before stimulation with saliva (Figure 2G). Thus, despite the adherence-dependent partial shedding of l-selectin from neutrophils, saliva-induced NETosis was still l-selectin dependent in our experimental setup.
NADPH-oxidase and elastase are not required for saliva-induced NETosis that involves the MEK/ERK pathway
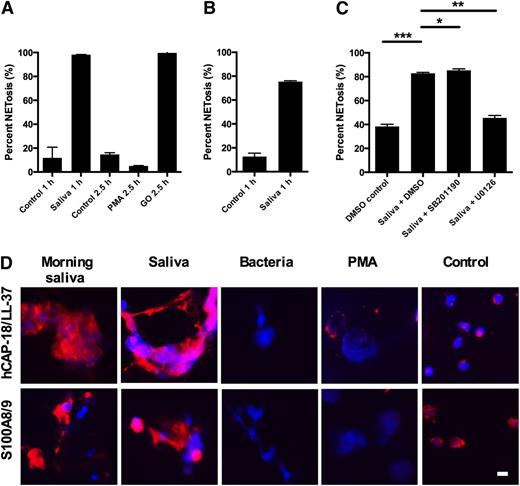
To investigate whether saliva-induced NETosis depends on reduced NAD phosphate (NADPH)-oxidase activity, experiments were performed with neutrophils from 2 patients with chronic granulomatous disease (CGD). CGD neutrophils are incapable of mounting a respiratory burst and did not form NETs in response to PMA, a potent activator of the NADPH oxidase in normal neutrophils. When H2O2 was provided by exogenous glucose oxidase, the CGD neutrophils formed NETs as expected (Figure 3A). CGD neutrophils formed NETs readily in response to saliva (Figure 3A), demonstrating that this l-selectin induced pathway is independent of the NADPH oxidase.
Saliva-induced NETosis is independent on NADPH oxidase or elastase and dependent on the MEK/ERK pathway. NETosis was stimulated in neutrophils and examined by immunofluorescence microscopy. NET formation was quantified as described in “Materials and methods.” Representative images are shown in supplementary Figure 3. (A) Neutrophils from 2 patients with chronic granulomatous disease (CGD) were stimulated with PMA or glucose oxidase (GO) for 2.5 hours or with saliva for 1 hour. Results are shown as the average from the 2 CGD patients. (B) Neutrophils from patient with PLS were stimulated for 1 hour with saliva. Results are shown as the average of 2 independent experiments with neutrophils PLS patient. (C) Neutrophils were preincubated with inhibitors or solvent (DMSO) before stimulation with saliva in the presence of inhibitors. Results are shown from 3 independent experiments. Scale bars, 100 μm. (D) Binding of various proteins to NETs isolated from morning saliva or induced with saliva, bacteria, or PMA. Control depicts nonstimulated neutrophils not undergoing NETosis. Columns denote average values and error bars standard deviations. Scale bars, 10 μm. *P < .05; **P < .01 and refer to nominal significance of inhibition or induction of NET formation.
Saliva-induced NETosis is independent on NADPH oxidase or elastase and dependent on the MEK/ERK pathway. NETosis was stimulated in neutrophils and examined by immunofluorescence microscopy. NET formation was quantified as described in “Materials and methods.” Representative images are shown in supplementary Figure 3. (A) Neutrophils from 2 patients with chronic granulomatous disease (CGD) were stimulated with PMA or glucose oxidase (GO) for 2.5 hours or with saliva for 1 hour. Results are shown as the average from the 2 CGD patients. (B) Neutrophils from patient with PLS were stimulated for 1 hour with saliva. Results are shown as the average of 2 independent experiments with neutrophils PLS patient. (C) Neutrophils were preincubated with inhibitors or solvent (DMSO) before stimulation with saliva in the presence of inhibitors. Results are shown from 3 independent experiments. Scale bars, 100 μm. (D) Binding of various proteins to NETs isolated from morning saliva or induced with saliva, bacteria, or PMA. Control depicts nonstimulated neutrophils not undergoing NETosis. Columns denote average values and error bars standard deviations. Scale bars, 10 μm. *P < .05; **P < .01 and refer to nominal significance of inhibition or induction of NET formation.
PMA and C. albicans induce NETosis by an elastase-dependent mechanism.10 Consequently, we investigated whether saliva could induce NETosis in neutrophils from a patient with Papillon-Lefévre syndrome (PLS). PLS neutrophils lack elastase and are incapable of forming NETs, both in response to PMA or glucose oxidase.18 In contrast, saliva induced robust NETosis in PLS neutrophils (Figure 3B).
l-selectin-mediated adhesion and degranulation is dependent on p38 MAPK.26 Preincubation of neutrophils with SB202190, an inhibitor of the MAPK pathway, and U0126, an inhibitor of the MEK/ERK pathway demonstrated that U0126, but not SB202190, inhibited saliva-induced NET formation (Figure 3C). Ten μM of U0126, used in these experiments, did not inhibit NETosis induced by PMA or by bacteria (supplementary Figure 3). This demonstrates that the intracellular signaling pathways in saliva-induced NETs are different from those in NETs induced by bacteria or PMA.
Due to the differences between saliva-induced NETs and other types of NETs, we examined the composition of proteins in the saliva-induced NETs in the oral cavity. NETs found in the oral cavity and NETs induced by saliva had more calgranulin and hCAP-18 bound than NETs induced by bacteria or PMA (Figure 4D).
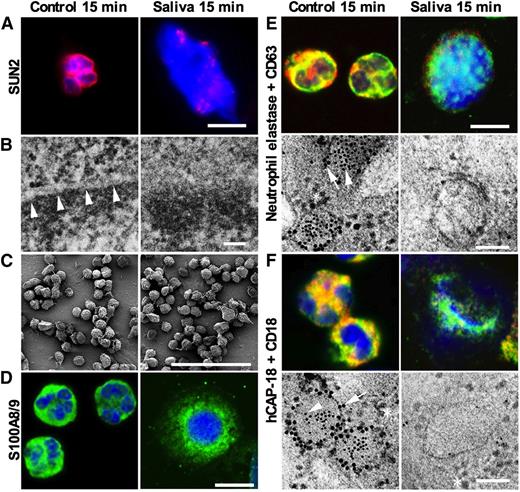
Intracellular event during saliva-induced NETosis. Neutrophils were left nontreated or were stimulated with saliva for 15 minutes and examined by immunofluorescence microscopy and electron microscopy. All fluorescence micrographs were acquired with a Zeiss LSM700 Axioimager M2 equipped with a 4-stack laser system (−405, −488, −555, −635 nm wavelength) using Zen (Zeiss) software, with a 63× oil immersion objective. Electron micrographs were acquired using a JEOL JEM 1230 transmission electron microscope and a JEOL JSM-350 scanning electron microscope with a secondary detector. (A) Immunofluorescence microscopy shows that staining for SUN2 found in the nuclear membrane is dramatically changed after 15 minutes stimulation by saliva. Scale bars represent 10 μm. (B) Transmission electron microscopy demonstrating that the nuclear membrane is lost after stimulation by saliva. Arrow heads indicate nuclear membrane. Scale bars, 50 nm. Larger images found in supplementary Figure 5. (C) Scanning electron microscopy of neutrophils demonstrating no visible changes in the plasma membrane after 15 minutes of saliva stimulation. Scale bars, 100 μm. Larger images found in supplementary Figure 4. (D) Confocal immunofluorescence microscopy for the cytosolic protein S100A8. Scale bars, 10 μm. (E) Confocal immunofluorescence microscopy and immunoelectron microscopy the azurophilic granule protein elastase (green or 5 nm gold particles) and CD63 (red or 10 nm gold particles) present in the azurophil granule membrane. Yellow color depicts colocalization. Many ribosomes of approximately the same size as the 10 nm gold particles are found around the granules in the electron micrograph. Arrow heads indicate elastase in the granule matrix and CD63 in the granule membrane. Scale bars, 10 μm for fluorescence micrographs and 100 nm for electron micrographs. (F) Confocal immunofluorescence microscopy and immunoelectron microscopy demonstrates the specific granule protein hCAP-18 (green or 5 nm gold particles) and CD18 (red or 10 nm gold particles) present in the membrane of specific granules. Yellow color depicts colocalization. Many ribosomes of approximately the same size as the 10 nm gold particles are found around the granules in the electron micrograph. Arrow heads indicate hCAP-18 in the granule matrix and CD18 in the granule membrane. Scale bars, 10 μm for fluorescence micrographs and 100 nm for electron micrographs.
Intracellular event during saliva-induced NETosis. Neutrophils were left nontreated or were stimulated with saliva for 15 minutes and examined by immunofluorescence microscopy and electron microscopy. All fluorescence micrographs were acquired with a Zeiss LSM700 Axioimager M2 equipped with a 4-stack laser system (−405, −488, −555, −635 nm wavelength) using Zen (Zeiss) software, with a 63× oil immersion objective. Electron micrographs were acquired using a JEOL JEM 1230 transmission electron microscope and a JEOL JSM-350 scanning electron microscope with a secondary detector. (A) Immunofluorescence microscopy shows that staining for SUN2 found in the nuclear membrane is dramatically changed after 15 minutes stimulation by saliva. Scale bars represent 10 μm. (B) Transmission electron microscopy demonstrating that the nuclear membrane is lost after stimulation by saliva. Arrow heads indicate nuclear membrane. Scale bars, 50 nm. Larger images found in supplementary Figure 5. (C) Scanning electron microscopy of neutrophils demonstrating no visible changes in the plasma membrane after 15 minutes of saliva stimulation. Scale bars, 100 μm. Larger images found in supplementary Figure 4. (D) Confocal immunofluorescence microscopy for the cytosolic protein S100A8. Scale bars, 10 μm. (E) Confocal immunofluorescence microscopy and immunoelectron microscopy the azurophilic granule protein elastase (green or 5 nm gold particles) and CD63 (red or 10 nm gold particles) present in the azurophil granule membrane. Yellow color depicts colocalization. Many ribosomes of approximately the same size as the 10 nm gold particles are found around the granules in the electron micrograph. Arrow heads indicate elastase in the granule matrix and CD63 in the granule membrane. Scale bars, 10 μm for fluorescence micrographs and 100 nm for electron micrographs. (F) Confocal immunofluorescence microscopy and immunoelectron microscopy demonstrates the specific granule protein hCAP-18 (green or 5 nm gold particles) and CD18 (red or 10 nm gold particles) present in the membrane of specific granules. Yellow color depicts colocalization. Many ribosomes of approximately the same size as the 10 nm gold particles are found around the granules in the electron micrograph. Arrow heads indicate hCAP-18 in the granule matrix and CD18 in the granule membrane. Scale bars, 10 μm for fluorescence micrographs and 100 nm for electron micrographs.
Saliva induces rapid loss of nuclear membrane and release of granule content intracellularly prior to NET-release
To investigate the nuclear events in saliva-induced NET formation, immunofluorescence microscopy was performed with focus on the nuclear membrane protein SUN2. Perinuclear SUN2 staining was found in nonstimulated polymorphonuclear leukocytes, whereas the perinuclear staining was replaced by disordered nuclear staining of SUN2 and nuclear swelling as early as 15 minutes after saliva stimulation, indicating disruption of the nuclear membrane (Figure 4A). Transmission electron microscopy confirmed the loss of nuclear membrane 15 minutes after saliva stimulation (Figure 4B), whereas the plasma membrane remained intact judged by scanning electron microscopy (Figure 4C). Confocal immunofluorescence microscopy demonstrated colocalization of the cytosolic protein S100A8 and DNA 15 minutes after saliva stimulation (Figure 4D), demonstrating that the loss of nuclear membrane is accompanied by access of cytosolic proteins to the DNA. To investigate the dynamics of neutrophil granules in saliva-induced NETosis, electron microscopy and confocal microscopy were performed to identify markers of azurophil granule matrix proteins and membrane proteins (elastase and CD63, respectively) and specific granule matrix proteins and membrane proteins (hCAP-18 and CD18, respectively). Although the granules seemed intact 15 minutes after onset of saliva stimulation there was no colocalization of elastase and CD63 or of hCAP-18 and CD18 (Figure 4E-F), demonstrating intracellular release of granule matrix proteins prior to extracellular release of DNA.
Saliva NETs are more DNase resistant than NETs induced by PMA or bacteria
The finding of NETs in the oral cavity was surprising, given the well-known DNase activity present in saliva.27,28 Accordingly, we investigated the DNase resistance of saliva-induced NETs compared with NETs induced by PMA or bacteria. Although saliva-induced NETs were resistant to the DNase activity of saliva, NETs induced by PMA or by bacteria were readily degraded (Figure 5A). To investigate the susceptibility of bacterial DNases, NETs were incubated with supernatant from S. pyogenes with DNase activity. A concentration of bacterial supernatant that caused complete degradation of NETs induced by PMA or by bacteria caused only minimal degradation of saliva-induced NETs (Figure 5A). Although NETs induced by sialyl LewisX in RPMI 1640 medium instead of saliva buffer were readily degraded by both saliva and AP-1 supernatant (Figure 5B), NETs induced by sialyl LewisX in saliva buffer were DNase resistant. Immunofluorescence microscopy demonstrated increased binding of actin, a known DNase inhibitor, to the DNA of saliva-induced NETs and NETs induced by sialyl LewisX in saliva buffer compared with NETs induced by sialyl LewisX in RPMI 1640, indicating that the hypotonicity of saliva promoted binding of actin (supplementary Figure 5). Only a minor difference in DNase resistance and actin binding was seen in NETs induced by PMA in saliva buffer compared with in RPMI 1640 (Figure 5B; supplementary Figure 5), demonstrating that the ionic composition of saliva, combined with the distinct mechanism of saliva/sialyl LewisX-induced NETosis, is responsible for the increased actin binding to DNA and DNase resistance.
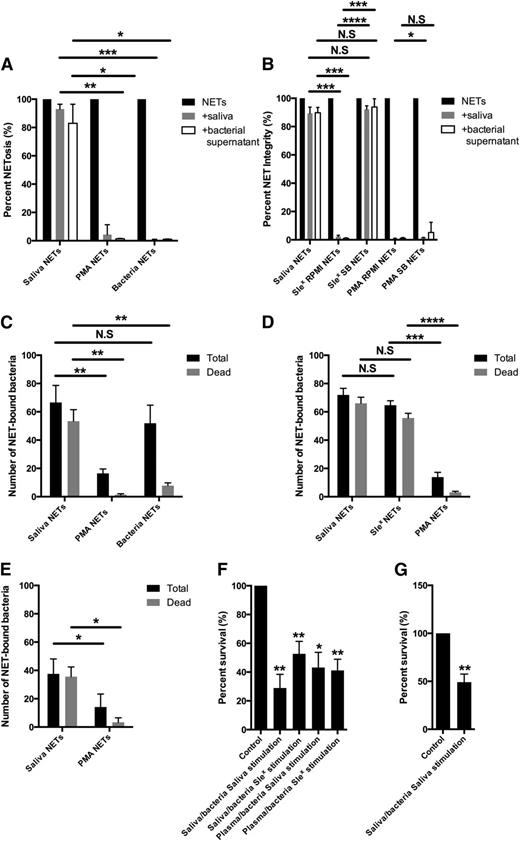
Properties of saliva-induced NETs. (A) NETs were induced with saliva, PMA, or bacteria. Microscopy images were taken to determine the area of NETs. The NETs were subsequently incubated with saliva or supernatant from S. pygogenes (AP1) with DNase activity and the area of NETs after treatment was determined by microscopy. The area of NETs before degradation was set to 100. Results are shown from 3 independent experiments. Representative images are found in supplementary Figure 5. (B) NETs were induced by saliva, sialyl LewisX in RPMI 1640 or saliva buffer (SB), and PMA in RPMI 1640 or SB. NET degradation was subsequently quantitated after treatment with saliva or AP1 supernatant. Results are shown from four independent experiments quantified as in A. (C) NETs were induced by saliva, PMA or bacteria and subsequently incubated with S. aureus. The total amount and amount of dead bacteria were examined by bacterial viability staining. Quantitative data from three independent experiments are presented. Representative images are found in supplementary Figure 5. (D) NETs were induced by saliva, sialyl LewisX and PMA and subsequently incubated with S. aureus. The total amount and amount of dead bacteria were examined by bacterial viability staining. Quantitative data from four independent experiments are presented. (E) NETs were induced by saliva and PMA and subsequently incubated with oral bacteria cultured from saliva. The total amount and amount of dead bacteria were determined by bacterial viability staining. Quantitative data from four independent experiments are presented where the oral bacteria were from 4 different donors. (F) Neutrophils were stimulated to phagocytose S. pyogenes either opsonized with saliva (saliva-opsonized bacteria SB) or plasma (plasma-opsonized bacteria). Extracellular bacteria were killed by gentamycin. NETosis was induced by saliva or sialyl LewisX. The NETs were disrupted by high concentration of DNase and colony counts performed on cell lysates. The survival of bacteria in polymorphonuclear leukocytes not stimulated to NETosis was set to 100. Results are shown from 4 independent experiments. (G) Similar experiments (as in F) were performed with oral bacteria cultured from saliva and opsonized with saliva prior to phagocytosis. Results are shown from 4 independent experiments in which oral bacteria were from 4 different donors. Columns denote average values and error bars standard deviations. N.S., not significant. *P < .05; **P < .01; ***P < .001; ****P < .0001 refers to nominal significance.
Properties of saliva-induced NETs. (A) NETs were induced with saliva, PMA, or bacteria. Microscopy images were taken to determine the area of NETs. The NETs were subsequently incubated with saliva or supernatant from S. pygogenes (AP1) with DNase activity and the area of NETs after treatment was determined by microscopy. The area of NETs before degradation was set to 100. Results are shown from 3 independent experiments. Representative images are found in supplementary Figure 5. (B) NETs were induced by saliva, sialyl LewisX in RPMI 1640 or saliva buffer (SB), and PMA in RPMI 1640 or SB. NET degradation was subsequently quantitated after treatment with saliva or AP1 supernatant. Results are shown from four independent experiments quantified as in A. (C) NETs were induced by saliva, PMA or bacteria and subsequently incubated with S. aureus. The total amount and amount of dead bacteria were examined by bacterial viability staining. Quantitative data from three independent experiments are presented. Representative images are found in supplementary Figure 5. (D) NETs were induced by saliva, sialyl LewisX and PMA and subsequently incubated with S. aureus. The total amount and amount of dead bacteria were examined by bacterial viability staining. Quantitative data from four independent experiments are presented. (E) NETs were induced by saliva and PMA and subsequently incubated with oral bacteria cultured from saliva. The total amount and amount of dead bacteria were determined by bacterial viability staining. Quantitative data from four independent experiments are presented where the oral bacteria were from 4 different donors. (F) Neutrophils were stimulated to phagocytose S. pyogenes either opsonized with saliva (saliva-opsonized bacteria SB) or plasma (plasma-opsonized bacteria). Extracellular bacteria were killed by gentamycin. NETosis was induced by saliva or sialyl LewisX. The NETs were disrupted by high concentration of DNase and colony counts performed on cell lysates. The survival of bacteria in polymorphonuclear leukocytes not stimulated to NETosis was set to 100. Results are shown from 4 independent experiments. (G) Similar experiments (as in F) were performed with oral bacteria cultured from saliva and opsonized with saliva prior to phagocytosis. Results are shown from 4 independent experiments in which oral bacteria were from 4 different donors. Columns denote average values and error bars standard deviations. N.S., not significant. *P < .05; **P < .01; ***P < .001; ****P < .0001 refers to nominal significance.
Antibacterial activity of saliva-induced NETs
Due to the increased binding of antibacterial proteins from cytosol and specific granules (calgranulin and hCAP-18, respectively) to saliva-induced NETs, we compared the capacity of saliva-induced NETs with NETs induced by PMA and bacteria to bind and kill NET-bound S. aureus. Saliva-induced NETs bound a larger number of bacteria than NETs induced by PMA (Figure 5C). Live/dead staining of NET-bound bacteria revealed a significantly higher percentage of dead bacteria in saliva-induced NETs compared with both PMA or bacteria-induced NETs (Figures 5C). NETs induced by sialyl LewisX bound and killed bacteria to a similar extent as saliva-induced NETs and significantly more than PMA-induced NETs (Figure 5D). Consequently, the capacity of saliva-induced NETs to bind and kill bacteria is not due to the binding of saliva components to the NETs, but to the distinct NETosis induced by saliva, resulting in binding of more neutrophil-derived antimicrobial proteins compared with the other types of NETs. The antibacterial effects of saliva induced NETs on oral bacteria was investigated next. Similar to the experiments with S. aureus, live/dead staining of NET-bound bacteria demonstrated that saliva-induced NETs bound significantly more bacteria cultivated from saliva than PMA-induced NETs, and most of the oral bacteria bound to saliva-induced NETs were dead (Figure 5E).
Streptococcus pyogenes survives inside the neutrophil phagosome,29 probably by inhibiting fusion of azurophil granules with the phagosome.30 To study the fate of intracellular bacteria in neutrophils undergoing saliva-induced NETosis, neutrophils were stimulated to phagocytose S. pyogenes (AP1). Nonphagocytosed extracellular bacteria were killed with gentamycin, and neutrophils were stimulated by saliva or sialyl LewisX. This caused a 50% to 70% reduction in the number of live intracellular bacteria (Figure 5F). Experiments with oral bacteria cultured from saliva demonstrated similar decrease in intracellular survival of bacteria in neutrophils when stimulated by saliva (Figure 5G). Neutrophils with phagocytosed bacteria underwent NETosis rapidly within 20 minutes of stimulation by saliva, but killing of intracellular bacteria after stimulation by saliva could be observed prior to NET release (supplementary Figure 5).
Ability of saliva-induced NETs to activate the complement system
PMA-induced NETs are known to activate the complement system.31 We found that PMA-induced NETs avidly bound complement component C3 both from normal human serum and from heat-inactivated serum (supplementary Figure 5D). However, little C3 was found bound to saliva-induced NETs and NETs from the oral cavity, demonstrating that presence of saliva-induced NETs at the oral mucosal surface does not result in complement activation (supplementary Figure 5E).
Saliva-induced NETosis is deficient in patients with Behçet disease and recurrent aphthous ulcerations
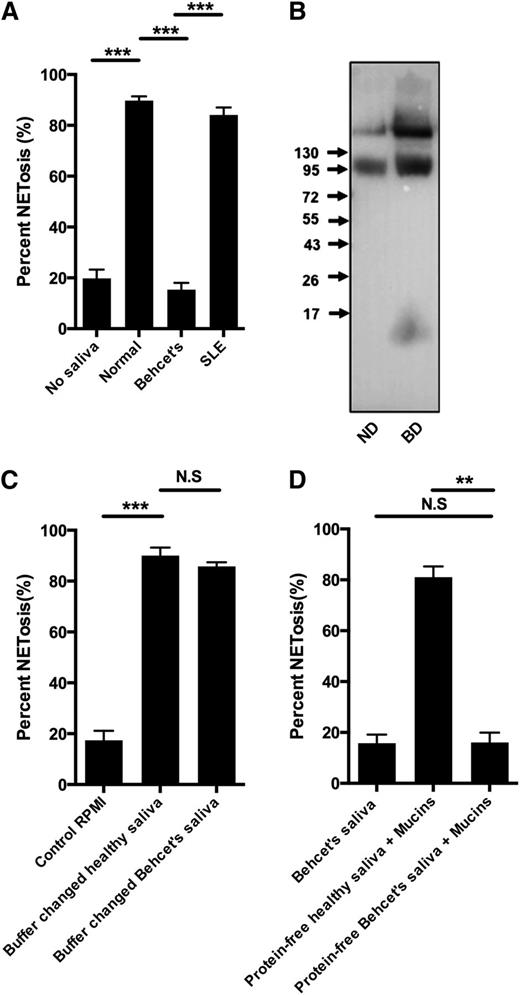
Behçet disease is a condition of unknown etiology, but it is characterized by ulcerations of mucous membranes with oral ulcers as a defining feature.32 Although saliva from 5 patients with Behçet disease failed to induce NETosis (Figure 6A), saliva from 5 patients with systemic lupus erythematosus (SLE) also receiving anti-inflammatory medication induced NETs similar to saliva from healthy donors (Figure 6A). Most patients with Behçet disease received colchicine (supplementary Table 1 for medication taken by patients with Behçet disease and SLE). However, saliva from a colchicine-treated patient who did not have Behçet disease, induced NETosis and colchicine did not inhibit saliva-induced NETosis (supplementary Figure 6). Western blot demonstrated a similar presence of sialyl LewisX in saliva from patients with Behçet disease and healthy controls (Figure 6B). Buffer changed saliva from patients with Behçet disease-induced NETosis (Figure 6C), whereas mucins diluted in protein-free Behçet saliva failed to induce NETosis, demonstrating that substance(s) in the protein-free fraction of Behçet saliva inhibit(s) the saliva-induced NETosis (Figure 6D) through interactions with mucins.
Deficient saliva-induced NETosis in response to saliva from patients with Behçet disease due to composition of protein-free fraction of saliva. Neutrophils were stimulated to NETosis and examined by immunofluorescence microscopy and NET formation quantified as described in “Materials and methods.” Representative images are shown in supplementary Figure 6. (A) Neutrophils were stimulated with saliva from 5 patients with Behçet disease, 5 patients with SLE or with saliva from normal donors. (B) Western blot of sialyl LewisX in saliva from normal healthy donor (ND) and patient with Behçet’s disease (BD). The same pattern was found in all samples of patients with Behçet disease. (C) NETosis of neutrophils stimulated with buffer changed saliva from ND and BD patients. (D) NETosis of neutrophils stimulated with saliva from BD patients, mucins resuspended in protein-free saliva from ND patients, and protein-free saliva from BD patients. Representative images are found in supplementary Figure 6. Columns denote average values and error bars standard deviations. N.S., not significant. **P < .01; *** P < .001.
Deficient saliva-induced NETosis in response to saliva from patients with Behçet disease due to composition of protein-free fraction of saliva. Neutrophils were stimulated to NETosis and examined by immunofluorescence microscopy and NET formation quantified as described in “Materials and methods.” Representative images are shown in supplementary Figure 6. (A) Neutrophils were stimulated with saliva from 5 patients with Behçet disease, 5 patients with SLE or with saliva from normal donors. (B) Western blot of sialyl LewisX in saliva from normal healthy donor (ND) and patient with Behçet’s disease (BD). The same pattern was found in all samples of patients with Behçet disease. (C) NETosis of neutrophils stimulated with buffer changed saliva from ND and BD patients. (D) NETosis of neutrophils stimulated with saliva from BD patients, mucins resuspended in protein-free saliva from ND patients, and protein-free saliva from BD patients. Representative images are found in supplementary Figure 6. Columns denote average values and error bars standard deviations. N.S., not significant. **P < .01; *** P < .001.
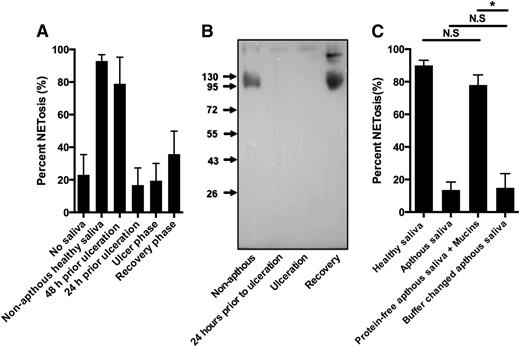
We also tested saliva from 2 otherwise healthy persons with recurrent oral aphthous ulcers (aphthae), a disease with recurrent formation of noncontagious painful mouth ulcers of unknown etiology.33 NETs formed readily in response to saliva collected from aphthae-free periods, but saliva from periods with aphthae had reduced ability to form NETs corresponding to the severity of the aphthous ulcers. In one case, failure of saliva to induce NETosis preceded the occurrence of oral ulcers (Figure 7A; supplementary Figure 7A). In the persons with aphthous stomatitis the failure of saliva to induce NETs was paralleled by loss of immunoreactivity for sialyl LewisX (Figure 7B; supplementary Figure 7). Buffer change did not restore the capability of the aphthous saliva to induce NETosis and mucins diluted in protein-free aphthous saliva readily induced NETs (Figure 7C), demonstrating that the protein-free aphthous saliva did not contain substances that inhibited mucin-mediated NETosis. Instead, the deficiency of aphthous stomatitis saliva to induce NETosis was paralleled by the loss of sialyl LewisX.
Deficient saliva-induced NETosis in response to saliva from patients with recurrent aphthous stomatitis due to loss of saliva sialyl LewisX. Neutrophils were stimulated to NETosis and examined by immunofluorescence microscopy and NET formation quantified as described in “Materials and methods.” Representative images are shown in supplementary Figure 7. (A) Neutrophils were stimulated with saliva from a person with recurrent aphthous stomatitis. Saliva was collected from a nonaphthous period, just prior to ulceration, from a period with ulceration, and during the recovery phase in which ulcers were still present. Error bars indicate standard deviation between 20 random images taken from each condition. Representative images and data from another person with recurrent aphthous ulcers are shown in supplementary Figure 7. (B) Western blot of sialyl LewisX in saliva from patient with recurrent aphthous stomatitis. (C) NETosis of neutrophils stimulated with saliva from healthy donor and saliva, buffer changed saliva, and mucins resuspended in protein-free saliva from a patient with recurrent aphthous stomatitis during the aphthous phase. N.S., not significant. *P < .05.
Deficient saliva-induced NETosis in response to saliva from patients with recurrent aphthous stomatitis due to loss of saliva sialyl LewisX. Neutrophils were stimulated to NETosis and examined by immunofluorescence microscopy and NET formation quantified as described in “Materials and methods.” Representative images are shown in supplementary Figure 7. (A) Neutrophils were stimulated with saliva from a person with recurrent aphthous stomatitis. Saliva was collected from a nonaphthous period, just prior to ulceration, from a period with ulceration, and during the recovery phase in which ulcers were still present. Error bars indicate standard deviation between 20 random images taken from each condition. Representative images and data from another person with recurrent aphthous ulcers are shown in supplementary Figure 7. (B) Western blot of sialyl LewisX in saliva from patient with recurrent aphthous stomatitis. (C) NETosis of neutrophils stimulated with saliva from healthy donor and saliva, buffer changed saliva, and mucins resuspended in protein-free saliva from a patient with recurrent aphthous stomatitis during the aphthous phase. N.S., not significant. *P < .05.
Discussion
NETs can be induced either through NADPH oxidase activation and elastase activity7 or by bacteria/bacterial products through a combination of signals from Toll-like receptors and integrins, either TLR4 and CD11a/CD188 or TLR2 and CR3 (CD11b/CD18).14 We found NETs in the oral cavity with a profile of associated proteins different from these NETs. These distinct oral NETs were induced by a novel mechanism for NET formation elicited by sialyl LewisX – l-selectin signaling. Sialyl LewisX mediates binding of l-selectin to endothelial cells, and this promotes rolling of neutrophils and activation of β2-integrins.24 However, the binding of l-selectin to sialyl LewisX is of low affinity.34 When neutrophils were stimulated on coverslips, the lowest concentration of sialyl LewisX capable of inducing significant NETosis was 5 μg/mL corresponding to 6 μM. This indicates that the concentration of sialyl LewisX necessary to induce NETs is significantly higher than that to which neutrophil are exposed during rolling on activated endothelium.
In contrast to NADPH oxidase-dependent NETosis induced by PMA, saliva-induced NET formation occurs more rapidly and is independent of NADPH oxidase and elastase as evidenced by experiments with CGD and PLS neutrophils. In contrast to bacteria-induced NETosis, the saliva-induced NETosis is independent of integrins. NETosis induced by bacteria and saliva occurs rapidly, but with marked differences in the intracellular nuclear events. In bacteria-induced NETs, extracellular DNA release is seen already after 10 minutes.13 The bacteria-induced NETs are formed by nuclear budding with dissociation between the inner and outer nuclear membrane with vesicular NET release from the nucleus. Accordingly, intact nuclear membrane can be observed together with extracellular DNA13 at early time points during NET release. It is only at later stages that the nuclear membrane is disrupted.13 This mechanism of NETosis is termed vital NETosis8 and results in nuclei-free cells capable of chemotaxis.14 In contrast, saliva-induced NET formation involves a complete disruption of the nuclear membrane with nuclear swelling at an early stage prior to any release of extracellular DNA with no evidence of nuclear budding or vesicular DNA release.
Elastase is released from azurophil granules during PMA-induced NETosis neutrophil35 by a mechanism supposedly similar to the release of cathepsins from lysosomes during autophagy.36 However, no egress of matrix proteins from specific granules has been described. Granule proteins were intracellularly present prior to DNA release during saliva-mediated NETosis, but neither elastase nor hCAP-18 was found in granules. Normally, neutrophil granules release their content by fusion with the phagosome or the plasma membrane,37 but this did not seem to occur during saliva-induced NETosis because we did not observe translocation of granule membrane proteins to other organelles. To our knowledge, such intracellular release of neutrophil granule content has not been previously described. We found almost total loss of immunologic reactivity of granule membrane proteins, indicating that the intracellular release of granule contents involves degradation of granule membrane proteins in both azurophil and specific granules. We observed increased killing of intracellular bacteria in saliva-stimulated neutrophils prior to NET release, but after intracellular release of granule proteins. It is, thus, tempting to hypothesize that the intracellular release of antimicrobial granule proteins contributed to the increased intracellular bacterial killing.
Investigating the antimicrobial function of NETs by colony counts is challenging8 because it is impossible to exclude bacteria killed by phagocytosis and bacteria not bound to NETs from the assays. Accordingly, conflicting data have been presented regarding the antimicrobial function of NETs.7,38 We cultured bacteria under conditions to obtain >99% live bacteria that were then incubated with NETs and subsequently stained with dyes reflecting bacterial viability. This allowed evaluation of both number and viability of bacteria bound to individual NETs, making it possible to exclude bacteria not bound to NETs and bacteria killed by phagocytosis. This revealed that saliva-induced NETs bound a larger number of bacteria than PMA-induced NETs and that significantly more of the NET-bound bacteria were killed by saliva-induced NETs compared with NETs induced by PMA or by bacteria. Increased binding of antimicrobial proteins from cytosol and specific granules paralleled the increased bactericidal activity of saliva-induced NETs compared with other types of NETs. Importantly, NETs induced by isolated sialyl LewisX had similar capacity to bind and kill bacteria as saliva-induced NETs. Therefore, it is the distinct NETs induced by sialyl LewisX, whether isolated or as part of salival mucins and not components present in saliva per se that is responsible for the capacity of saliva-induced NETs to bind and kill bacteria.
Neutropenia and functional neutrophil deficiencies are associated with disordered homeostasis in the oral cavity manifested, for example, by oral ulcerations.5 We demonstrated deficient saliva-induced NET formation in 2 conditions of unknown etiology, but with normal neutrophil count and with oral ulcers as a defining feature, recurrent aphthous stomatitis, and Behçet disease.33,39 Indeed, saliva from patients with both conditions failed to induce NETs, but for different reasons. In recurrent aphthous stomatitis, the mucins lost the ability to induce NETs due to loss of sialyl LewisX. Possibly bacterial sialidases produced by the oral flora40 contribute to the loss of sialyl LewisX in salival mucins in recurrent aphthous stomatitis. In contrast, sialyl LewisX was present on salival mucins in Behçet disease. In Behçet disease, components in the protein-free fraction of saliva (saliva fluid) inhibited the ability of the mucins to induce NETs. Because sialyl LewisX/saliva mediated neutrophil killing of bacteria from normal oral flora, it is interesting to note that the oral flora is altered in both recurrent aphthous stomatitis and Behçet disease.39,41
Examination of just a few parameters: binding of complement components, DNase resistance, and the capacity to bind and kill bacteria, revealed that different mechanisms of NETosis lead to functionally distinct NETs. Although NETs have been implicated in the development of diseases such as nephritis and thrombosis,16,17 our study suggests that NETs with antimicrobial activity represent a normal fate of neutrophils in the oral cavity, important for a healthy oral mucosal surface. Future studies will determine whether mucins at other mucosal surfaces interact with neutrophils and thereby influence neutrophil fate by inducing NETosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Malgorzata Berlikowski, Maria Baumgarten, and Charlotte Horn for their expert technical assistance; Azra Kurbasic for dedicated statistical support; Lars Björck for useful discussions and pivotal support, Mads Nybo for providing crucial laboratory facilities for processing of patient samples, and Maria Andersson, Anita Nihlberg, and Robin Kahn for dedicated help in collection of patient saliva samples.
This work was supported by grants from the Alfred Österlund Foundation, the Petrus and Augusta Hedlund Foundation, the Royal Physiographic Society Lund, the Greta och Johan Kock Foundation, the Crafoord Foundation, and the Danish Medical Research Council.
Author contributions
Contribution: T.M., J.S., M.M., and O.S. designed experiments; T.M., J.S., A.H., and M.M. performed experiments; F.K. analyzed and collected clinical material; N.F., K.A., A.B., and N.B. analyzed and provided clinical samples; T.M., N.B., and O.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ole E. Sørensen, Division of Infection Medicine, Nuclear Biology Laboratory, Biomedical Center, B14, Department of Clinical Sciences, Lund University, Tornavägen 10, SE-221 84 Lund, Sweden; e-mail: ole_e.sorensen@med.lu.se.