Key Points
CD8 memory T cells in PBMCs are antigen-hyporesponsive due to loss of priming by tissue-dependent interactions.
Preculture at high cell density allows the detection of antiviral and antitumor responses that may be overlooked without this step.
Abstract
Peripheral blood mononuclear cells (PBMCs) are the only source of human lymphoid cells routinely available for immunomonitoring of T-cell responses to microbial and tumor-associated antigens. However, previous work in mice and humans had indicated that CD4 T cells transiently lose antigen sensitivity when cellular contacts are lost (eg, by entering the circulation). Using the simple and robust protocol for resetting T cells to original reactivity (RESTORE; ie, preculturing PBMCs for 2 days at a high cell density before initiation of antigenic stimulation), we show that CD8 T-cell responses to viral and tumor-associated antigens are greatly underestimated in blood, and sometimes even remain undetected, if conventional, unprocessed PBMC cultures are used. The latter finding is particularly striking with regard to the appearance of Wilms tumor 1 protein-specific CD8 T-cell responses in leukemia patients after allogeneic bone marrow transplantation. The dramatic increase in antigen sensitivity of “restored” CD8 T cells is associated with phosphorylation of proximal T-cell receptor signaling components, and with the upregulation of genes involved in aerobic glycolysis, thereby increasing T-cell functionality. The RESTORE protocol permits a more meaningful monitoring of CD8 memory T-cell responses to viral infections and tumors and vaccination success. Furthermore, when generating T-cell lines for adoptive T-cell therapy, it avoids the loss of those clones, which strictly depend on the primed status conferred by cellular interactions in the tissue context for their initial reactivation by antigen. The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE63430).
Introduction
CD8 T cells specific for viral or tumor antigens can either be detected by HLA/peptide multimer staining, an option only available at considerable cost and requiring knowledge of the relevant allelic HLA molecules and antigenic peptides involved, or by the detection of functional responses to peptide pools, usually the production of interferon (IFN)-γ.
T cells actively search for major histocompatibility complex (MHC)/peptide complexes displayed on the surfaces of other cells, which can either be specialized antigen-presenting cells (APC) during the priming phase, or almost any other cell type of the body during immune surveillance.1-3 This search for antigens is performed by a crawling process involving integrin-mediated adhesion and cytoskeletal reorganization,4-6 and by the interaction of T-cell receptor (TCR) with MHC molecules,7,8 both of which prime T cells for recognition of cognate MHC/peptide complexes by presensitizing the signaling machinery.1,4,9
When T cells interrupt their search for antigens in the tissue context to travel to a different body site via the circulation, they transiently live as dispersed cells without cellular contacts and, consequently, lose their preactivated status.1,9-11
An extreme case illustrating this phenomenon is the dramatic difference in the responses of tissue-resident vs peripheral blood mononuclear cell (PBMC)-derived human T cells to the CD28 superagonist TGN1412 (now called TAB08).12 Although PBMC cultures fail to respond to this monoclonal Ab (mAb) in soluble form,13 its application to healthy volunteers in a phase 1 study in 2006 resulted in a life-threatening cytokine release syndrome.14 The molecular basis for this effect is the strict dependence of CD28-driven T-cell activation on a preactivated TCR signaling machinery that is amplified by the co-stimulatory signal at the level of the SLP76 signalosome.15 Based on the unexpected observation that strong TGN1412 reactivity is restored by simply culturing PBMCs for 2 days at a high density (HD), we could indeed demonstrate that a phase of cell-cell contacts involving MHC scanning is required to reset circulating T cells to a tissue-like status, thereby allowing superagonist-ligated CD28 molecules to trigger T-cell activation.11
A “tissue-like” in vitro response of CD8 T cells to viral and tumor-associated antigens would be highly desirable for immunomonitoring, and could improve the generation of CD8 T-cell lines and clones for cellular immunotherapy. Therefore, we have now asked the question whether HD preculture also allows more sensitive detection of CD8 T-cell responses. We report that this is indeed the case, and, in some instances, resetting circulating T cells to tissue-like conditions even reveals CD8 T-cell responses that would otherwise go undetected.
Materials and methods
PBMCs
Human PBMCs were prepared from healthy donors from leukoreduction system chambers16 or as heparinized venous blood from patients who had undergone myeloablation and allogeneic hematopoietic stem cell transplantation (HSCT) and were in complete hematologic remission. PBMCs were obtained by density gradient centrifugation and kept on ice until use within 1 hour, or freezing in 40% RPMI 1640 (Gibco), 50% AB-positive heat-inactivated human serum, and 10% dimethylsulfoxide (Sigma-Aldrich). The term “fresh” refers to freshly isolated PBMCs tested either directly or after freezing/thawing, which yielded identical results (see supplemental Figure 1C, available on the Blood Web site).
Tonsillar mononuclear cells (TMCs)
Uninfected fresh tonsils from children undergoing tonsillotomy at the University Hospital Würzburg were cut to small pieces, digested with collagenase IV (0.5 mg/mL) and DNase I (0.02 mg/mL; Sigma-Aldrich) at 37°C for 40 minutes, followed by filtration through a 45 μm mesh. Viable TMCs were isolated by density gradient centrifugation.
Cell culture and stimulation assays
Cells were cultured in supplemented AB medium11 at 37°C in 5% CO2. Conventional T-cell stimulation assays were either performed directly at low cell density (1 × 106 cells/mL) or upon application of the resetting T cells to original reactivity (RESTORE) protocol.11 In brief, PBMCs were precultured in media for 2 days at HD ( 1 × 107 cells/mL) to allow for tissue-like interactions. Functional responses were tested with PepMix CEF standard containing HLA-class I restricted T-cell epitopes from human cytomegalovirus (HCMV), Epstein-Barr virus (EBV) and influenza A virus, and PepMix influenza A. PepMix Human Actin served as negative control (JPT Peptide Technologies). Synthetic peptides from HCMV (PP65_HCMV 495-503), human adenovirus (ADV) 2 (HEX_ADE02 901-910, HEX_ADE02 37-45, and E1A_ADE02 19-27), and Wilms tumor 1 protein (WT1) (WT1_HUMAN 126-134 and WT1_HUMAN 356-364) were synthesized as previously described17 (purity >90%). Clinical grade TAB08 was used at 1 μg/mL. For isolation of cell subsets, human CD8 T-cell isolation kit and anti-human CD14-MicroBeads (Miltenyi Biotec) were used (purity >95%).
For in vitro expansion of antigen-specific CD8 T cells from fresh or precultured PBMCs, cells were stimulated at 2 × 106 cells/mL with 1 μg/mL of the peptide WT1_HUMAN 356-364 or 0.1 μg/mL of the PepMix CEF standard. On day 3, recombinant human interleukin (IL)-2 (50 U/mL; Chiron), IL-7, and IL-15 (10 U/mL; PeproTech) were added. Half-medium change and supplementation of cytokines were performed every 2 to 3 days until day 14.
IFN-γ enzyme-linked immunospot (ELISPOT) assay
ELISPOT assays were performed with MultiScreen 96-well plates at 2 × 105 cells/200 μL for 16 hours (Merck Millipore). Spots were determined by an ImmunoSpot S5 ELISPOT Analyzer (CTL).
Antibodies and flow cytometry
Western blotting
A total of 3 × 106 cells were lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich) supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted using antibodies to CD3ζ (6B10.2; Santa Cruz), phospho CD3ζ p-Y142 (K25-407.69; BD Biosciences), Lck, and phospho-Src family p-Y494 (polyclonal; Cell Signaling). ERK1/2 expression (polyclonal; Life Technologies) served as loading control.
Microarray analysis
High quality RNA was extracted from flow cytometry sorted CD8+CD45R0+ T cells from fresh or precultured PBMCs of 3 healthy individuals by the TRIzol method (Ambion). Samples were amplified using the ExpressArt mRNA Amplification Pico-Kit (Amptec). IVT-PLUS Kit (Affymetrix) was used for incorporation of biotin-labeled nucleotides. Samples were hybridized to PrimeView Human Gene Expression Arrays (Affymetrix). Raw signal intensities were normalized by variance stabilization.19 Statistical analysis was performed using the Linear Models of Microarray Analysis package.20 We required a nominal P < .05 and an absolute log2 fold-change of at least 1 between analysis groups. We used WebGestalt to search for enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.21
Statistics
Data are presented as mean ± standard deviation (SD) or median with interquartile range (IQR). Statistical significance was analyzed by unpaired Student t test, Wilcoxon signed-rank test, or Mann–Whitney U test, using GraphPad Prism Software 5.0d. Values of P < .05 were considered to be statistically significant.
Study approval
Studies using human material were approved by the University of Würzburg Committee on Human Research (study numbers 85/13 and 159/13) and by the Insitutional Review Board of the University of Würzburg. Informed consent was obtained in accordance with the guidelines of the World Medical Association’s Declaration of Helsinki. Written informed consent was received from donors prior to inclusion in the study.
Results
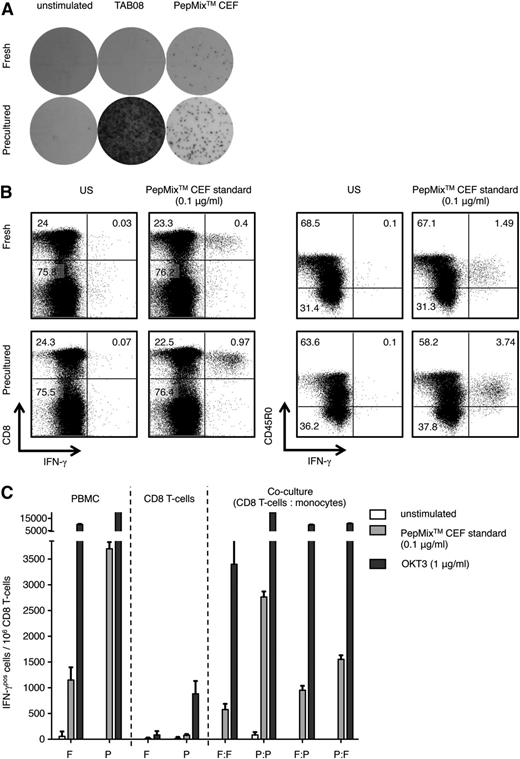
In a pilot experiment, we compared frequencies of CD8 T cells responding with IFN-γ release in an ELISPOT assay to 0.1 μg/mL of the HLA-class I restricted virus-specific PepMix CEF standard covering HCMV, EBV, and influenza between fresh PBMCs and PBMCs precultured according to the RESTORE protocol, developed for optimal detection of responses to the CD28 superagonist, TAB08,11 which was included as positive control. As seen in Figure 1A, HD preculture of human PBMCs strongly increased the frequency of detectable virus-specific CD8 T cells. Of note, the HD preculture step did not alter the frequency of CD8 T cells in the PBMC preparation11 (supplemental Table 1), indicating that their reactivity to the viral antigens had increased.
Enhanced antiviral IFN-γ response in human CD8 memory T cells by HD preculture of PBMCs. (A) Representative IFN-γ ELISPOT assay of fresh or precultured PBMCs. Cytokine release after stimulation with 1 μg/ml CD28 superagonist TAB08 (previously: TGN1412) and 0.1 μg/ml PepMix CEF standard was assessed after 16 hours. Unstimulated fresh and precultured PBMCs were used as negative controls. (B) Intracellular IFN-γ staining of fresh and precultured PBMCs after stimulation with 0.1 μg/ml PepMix CEF standard for 16 hours. Gating was performed on viable lymphocytes (left) and CD8 T cells (right). (C) Functional maturation of CD8 T cells and monocytes contributes to the HD preculture effect. CD8 T cells and monocytes were purified from fresh and precultured PBMCs of the same donor by magnetic sorting (magnetic-activated cells sorting) and cocultured under standard conditions at a 1:1 ratio in the presence of either 0.1 μg/ml PepMix CEF standard or 1 μg/ml OKT3, and were assessed by IFN-γ ELISPOT 16 hours after stimulation. Experiments were repeated ≥3 times. Data represent mean ± SD for triplicate samples for 1 representative donor out of 3. F, fresh; P, precultured; US, unstimulated.
Enhanced antiviral IFN-γ response in human CD8 memory T cells by HD preculture of PBMCs. (A) Representative IFN-γ ELISPOT assay of fresh or precultured PBMCs. Cytokine release after stimulation with 1 μg/ml CD28 superagonist TAB08 (previously: TGN1412) and 0.1 μg/ml PepMix CEF standard was assessed after 16 hours. Unstimulated fresh and precultured PBMCs were used as negative controls. (B) Intracellular IFN-γ staining of fresh and precultured PBMCs after stimulation with 0.1 μg/ml PepMix CEF standard for 16 hours. Gating was performed on viable lymphocytes (left) and CD8 T cells (right). (C) Functional maturation of CD8 T cells and monocytes contributes to the HD preculture effect. CD8 T cells and monocytes were purified from fresh and precultured PBMCs of the same donor by magnetic sorting (magnetic-activated cells sorting) and cocultured under standard conditions at a 1:1 ratio in the presence of either 0.1 μg/ml PepMix CEF standard or 1 μg/ml OKT3, and were assessed by IFN-γ ELISPOT 16 hours after stimulation. Experiments were repeated ≥3 times. Data represent mean ± SD for triplicate samples for 1 representative donor out of 3. F, fresh; P, precultured; US, unstimulated.
The elevated virus-specific IFN-γ response of precultured PBMCs relative to fresh PBMCs was confirmed by intracellular staining, in which CD8 memory cells (CD8+CD45R0+) were identified as the main cytokine source (Figure 1B).
We then tested whether the enhanced IFN-γ response of blood-derived CD8 memory T cells to the PepMix CEF standard requires interaction with matured monocytes that develop during the preculture step.11 CD8 T cells and monocytes were purified from fresh or precultured PBMCs, mixed in all combinations, and stimulated with the virus-specific peptide mix (Figure 1C). As reported above, CD8 T cells present in unseparated precultured PBMCs showed a strong increase in the frequency of responding cells compared with fresh PBMCs. Similarly high responses to the PepMix CEF standard were observed in 1:1 cocultures of CD8 T cells and monocytes if both had been purified from precultured PBMCs, but not if one of the partners was derived from fresh PBMCs. These results indicate that the functional maturation of both monocytes and CD8 memory cells during HD preculture contributes to improvement of peptide-specific responses. Of note, and in agreement with previously published data,11 the response to OKT3, which is a high-affinity ligand for the TCR complex, elicited strong responses in fresh and precultured PBMCs.
To optimize the conditions allowing improved CD8 T-cell responses, we next varied time (up to 3 days) and cell density (from 1 to 30 × 106 cells/mL) during the preculture period, and confirmed the conditions established in the original RESTORE protocol,11 ie, 2 days at 1 × 107 cells/mL (supplemental Figure 1A-B). We also excluded that the observed gain in reactivity is due to a loss of regulatory T cells during preculture by demonstrating their unchanged representation after this step, and unchanged reactivity of fresh PBMCs if regulatory T cells were removed prior to the assay (supplemental Figure 2). In addition, phenotypic analysis of CD8 T cells revealed that the representation of CD8 memory cells as well as their expression of cell surface molecules associated with co-stimulation or co-inhibition (CD28 and CTLA-4), apoptosis (Annexin V binding), or exhaustion (PD-1) remained unaffected by HD preculture, whereas glucocorticoid-induced tumor necrosis factor receptor expression increased and CD3 was down-modulated, possibly as a consequence of MHC scanning (supplemental Table 1). With regard to monocytes, the maturation of which also contributes to the improved reactivity of restored PBMCs (Figure 1C), we confirmed our previous observation that HLA molecules and the co-stimulatory ligand CD86 are upregulated by the preculture step.11
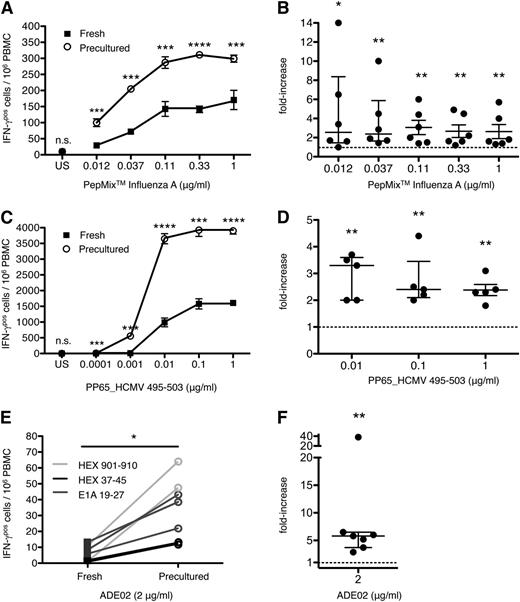
The effect of HD preculture on the sensitivity of the CD8 T-cell response was then quantified by titrating influenza and HCMV-derived peptides. HD preculture strongly increased the responsiveness of CD8 T cells to both viral antigens as exemplified in Figure 2A,C. The displacement of the titration curves of precultured compared with fresh PBMCs indicates a fivefold to 10-fold gain in sensitivity to the influenza A-derived peptide pool (Figure 2A), and a similar increase in sensitivity to PP65_HCMV 495-503 (Figure 2C). Here, PBMC preculture of a cytomegalovirus (CMV)–sero-positive patient allowed the detection of a CD8 T-cell response to as little as 0.001 μg/mL of peptide, whereas fresh cells failed to respond with IFN-γ secretion at that antigen concentration. The specificity of these responses, already apparent from their strict peptide dependence, was further proven by the complete lack of a response to PP65_HCMV 495-503 in fresh or precultured PBMCs from an HLA–A0201-positive, CMV–sero-negative patient (data not shown). A summary of results obtained with ≥5 donors is given in Figure 2B,D.
Enhanced sensitivity of human CD8 T cells to defined viral antigens by HD preculture of PBMCs. (A) IFN-γ responses of CD8 T cells from fresh or precultured PBMCs from a representative healthy donor were directly compared by ELISPOT assay after stimulation with titrated PepMix influenza A. Unpaired Student t test: ***P < .0005; ****P < .0001. (B) Compiled data from 6 healthy donors tested as in (A). Data represent the mean fold-increase in IFN-γ responses of CD8 T cells from precultured PBMCs relative to CD8 T cells from fresh PBMCs after stimulation with the PepMix influenza A. Mann–Whitney U test: *P < .05; **P < .01. Data represent median with IQR. Raw data: supplemental Figure 5A. (C) IFN-γ responses of CD8 T cells from fresh or precultured PBMCs from an HLA–A0201-positive, CMV–sero-positive healthy donor after stimulation with titrated PP65_HCMV 495-503. (C) Unpaired Student t test: ***P < .0005; ****P < .0001. (D) Compiled data from 5 donors tested as in (C). Data presentation as in (B). Fold increase could not be calculated for the concentrations 0.0001 and 0.001 μg/ml where no response was detectable in fresh PBMCs (supplemental Figure 5B). (E) CD8 T-cell responses in fresh and precultured PBMCs to 2 μg/ml of 3 different ADV-derived peptides. (F) Compiled data from 7 HLA-A01 (HEX_ADE02 901-910), HLA-A02 (E1A_ADE02 19-27), or HLA-A24 (HEX_ADE02 37-45) positive healthy individuals. Data presentation as in (B). Mann-Whitney U test: **P < .01. Data represent median with IQR. n.s., not significant.
Enhanced sensitivity of human CD8 T cells to defined viral antigens by HD preculture of PBMCs. (A) IFN-γ responses of CD8 T cells from fresh or precultured PBMCs from a representative healthy donor were directly compared by ELISPOT assay after stimulation with titrated PepMix influenza A. Unpaired Student t test: ***P < .0005; ****P < .0001. (B) Compiled data from 6 healthy donors tested as in (A). Data represent the mean fold-increase in IFN-γ responses of CD8 T cells from precultured PBMCs relative to CD8 T cells from fresh PBMCs after stimulation with the PepMix influenza A. Mann–Whitney U test: *P < .05; **P < .01. Data represent median with IQR. Raw data: supplemental Figure 5A. (C) IFN-γ responses of CD8 T cells from fresh or precultured PBMCs from an HLA–A0201-positive, CMV–sero-positive healthy donor after stimulation with titrated PP65_HCMV 495-503. (C) Unpaired Student t test: ***P < .0005; ****P < .0001. (D) Compiled data from 5 donors tested as in (C). Data presentation as in (B). Fold increase could not be calculated for the concentrations 0.0001 and 0.001 μg/ml where no response was detectable in fresh PBMCs (supplemental Figure 5B). (E) CD8 T-cell responses in fresh and precultured PBMCs to 2 μg/ml of 3 different ADV-derived peptides. (F) Compiled data from 7 HLA-A01 (HEX_ADE02 901-910), HLA-A02 (E1A_ADE02 19-27), or HLA-A24 (HEX_ADE02 37-45) positive healthy individuals. Data presentation as in (B). Mann-Whitney U test: **P < .01. Data represent median with IQR. n.s., not significant.
Since ADV-specific CD8 memory T cells are usually rare in peripheral blood, monitoring of patients at high risk for infection is difficult without prior in vitro expansion, which, however, falsifies their in vivo representation.22 We therefore investigated whether HD preculture of PBMCs also enhances the responsiveness of human ADV (ADE)-specific CD8 T cells to more readily detectable levels. Indeed, CD8 T cells from 7 tested healthy individuals showed strongly enhanced IFN-γ responses to the epitopes HEX 901-910, E1A 19-45, or HEX 37-45 from ADE02, the clinically important strain (Figure 2E-F).
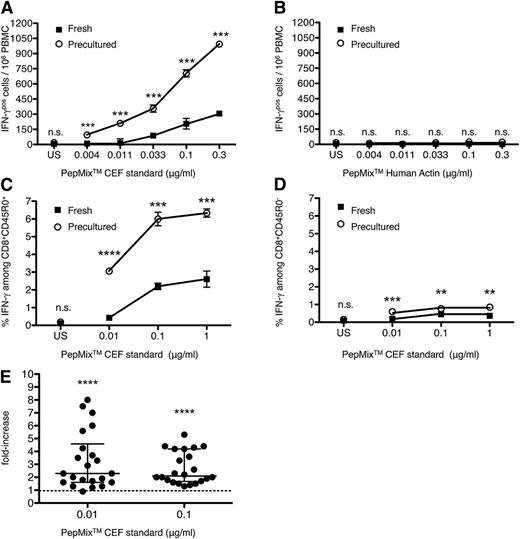
In order to obtain a larger data set allowing statistical assessment of the effects of HD preculture on the detection of antiviral responses, fresh or precultured PBMCs from 22 healthy individuals were stimulated with titrated amounts of PepMix CEF standard. An example of these responses is shown in Figure 3A, where CD8 T cells in precultured PBMCs stimulated with 0.033 μg/mL of the peptide pool showed an IFN-γ recall response comparable to that of CD8 T cells in fresh PBMCs stimulated with 0.3 μg/mL, indicating a 10-fold gain in sensitivity through the HD preculture step. No response was observed in response to the PepMix Human Actin regardless of the culture protocol (Figure 3B, same individual as in Figure 3A).
Enhanced recall responses of HD-precultured CD8 T cells to virus-derived peptide pools. (A) IFN-γ ELISPOT responses of fresh or precultured PBMCs of 1 representative healthy donor to titrated amounts of the HLA-class I restricted PepMix CEF standard. Unpaired Student t test: ***P < .0005. (B) Response of the same donor as shown in (A) to titrated amounts of the negative control pool PepMix Human Actin. Data represent mean ± SD for triplicate samples. (C-D) Identification of memory CD8 T cells as source for IFN-γ production by intracellular cytokine staining of fresh and precultured PBMCs of 1 representative donor that is different from (A-B). Results are shown as percentage of IFN-γ–positive cells among CD8+CD45R0+ cells and CD8+CD45R0− cells. (E) Compiled data from 22 healthy donors tested as in Figure 2A and presented as in Figure 2B. Mann-Whitney U test: ****P < .0001. Data represent median with IQR. Raw data: supplemental Figure 5C. n.s., not significant; US, unstimulated.
Enhanced recall responses of HD-precultured CD8 T cells to virus-derived peptide pools. (A) IFN-γ ELISPOT responses of fresh or precultured PBMCs of 1 representative healthy donor to titrated amounts of the HLA-class I restricted PepMix CEF standard. Unpaired Student t test: ***P < .0005. (B) Response of the same donor as shown in (A) to titrated amounts of the negative control pool PepMix Human Actin. Data represent mean ± SD for triplicate samples. (C-D) Identification of memory CD8 T cells as source for IFN-γ production by intracellular cytokine staining of fresh and precultured PBMCs of 1 representative donor that is different from (A-B). Results are shown as percentage of IFN-γ–positive cells among CD8+CD45R0+ cells and CD8+CD45R0− cells. (E) Compiled data from 22 healthy donors tested as in Figure 2A and presented as in Figure 2B. Mann-Whitney U test: ****P < .0001. Data represent median with IQR. Raw data: supplemental Figure 5C. n.s., not significant; US, unstimulated.
To test whether as expected, the increase in antigen-sensitivity observed over the whole titration range is a feature of the CD8 memory compartment, intracellular IFN-γ staining was performed with 1 representative donor (Figure 3C). Indeed, only memory CD8 T cells contributed to the improved response (Figure 3C-D).
Data compiled from all 22 individuals confirmed a significantly increased IFN-γ recall CD8 T-cell response to the PepMix CEF standard when HD precultured PBMCs were compared with freshly isolated cells (Figure 3E). The average fold-increase in the response of CD8 T cells from precultured relative to fresh PBMCs is presented as the median with IQR after stimulation with 0.01 μg/mL (2.3, IQR = 3.0) or 0.1 μg/mL (2.1, IQR = 2.5), and was found statistically highly significant. Although for some responders, little or no increase (but never a decrease) was seen after HD preculture, 5/22 individuals revealed 4× or higher increments at both peptide concentrations tested.
Loss of antiviral CD8 T-cell sensitivity in dispersed tissue cultures
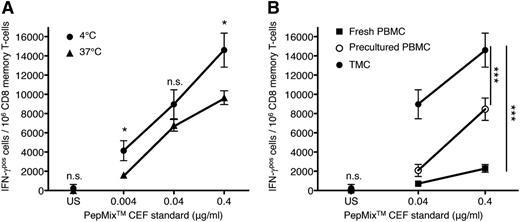
To gain support for our notion that the poor performance of fresh PBMCs compared with HD precultured ones reflects the restoration of antigen-sensitivity that was lost when leaving the tissue context to enter the circulation, TMCs from fresh uninfected tonsils were used as an available source of tissue-resident CD8 T cells. Initially, we compared the response of TMCs kept dispersed in suspension for 2 hours at 37°C (to simulate tissue exit) to that of cells kept on ice for the same time. Indeed, the interruption of cell contacts at body temperature resulted in a fivefold to 10-fold reduction in antigen sensitivity (see displacement of curves in Figure 4A) without loss in cell viability (not shown).
Loss of virus-specific CD8 T-cell reactivity in dispersed TMCs. (A) TMCs were prepared from uninfected fresh human tonsils and were either kept on ice or dispersed in suspension (1 × 106 cells/ml) for 2 hours at 37°C (5% CO2) to simulate tissue exit. IFN-γ responses to titrated amounts of the HLA-class I restricted PepMix CEF standard were analyzed by an IFN-γ ELISPOT assay, and the frequency of CD8 memory T cells in TMCs was determined by flow cytometry. Unpaired Student t test: *P < .05. (B) Virus-specific IFN-γ responses to 0.04 or 0.4 μg/ml of the peptide pool were compared between CD8 memory T cells from TMCs with those of fresh and HD precultured PBMCs of the same individual as presented in (A). Similar results were obtained after repetition of the experiment. Two-way analysis of variance, ***P < .0005. Data represent mean ± SD for triplicate samples. n.s., not significant.
Loss of virus-specific CD8 T-cell reactivity in dispersed TMCs. (A) TMCs were prepared from uninfected fresh human tonsils and were either kept on ice or dispersed in suspension (1 × 106 cells/ml) for 2 hours at 37°C (5% CO2) to simulate tissue exit. IFN-γ responses to titrated amounts of the HLA-class I restricted PepMix CEF standard were analyzed by an IFN-γ ELISPOT assay, and the frequency of CD8 memory T cells in TMCs was determined by flow cytometry. Unpaired Student t test: *P < .05. (B) Virus-specific IFN-γ responses to 0.04 or 0.4 μg/ml of the peptide pool were compared between CD8 memory T cells from TMCs with those of fresh and HD precultured PBMCs of the same individual as presented in (A). Similar results were obtained after repetition of the experiment. Two-way analysis of variance, ***P < .0005. Data represent mean ± SD for triplicate samples. n.s., not significant.
Furthermore, the ex vivo responsiveness of tonsillar CD8 memory T cells to PepMix CEF standard was compared with that of CD8 memory T cells in fresh and precultured PBMCs from the same individual (Figure 4B). As predicted from their previous cellular interactions in the tonsil tissue, CD8 memory T cells contained a much higher frequency (about 10-fold) of IFN-γ producers in response to 0.04 or 0.4 μg/mL of the viral peptide mix compared with fresh PBMCs. Although homing of virus-specific CD8 memory cells to this strategically important site may explain part of this huge difference, disablement by loss of tissue context during circulation seems to play an important role, as evidenced by the strong response of HD precultured PBMCs, which did, however, not reach that measured for TMCs of the same individual (Figure 4B).
Collectively, these results support the concept that cellular interactions within lymphoid tissues or tissue-like culture systems enable human CD8 memory T cells to respond effectively to antigens, a feature which is impaired during recirculation.
Facilitation of CD8 T-cell responses to the tumor-associated antigen WT1
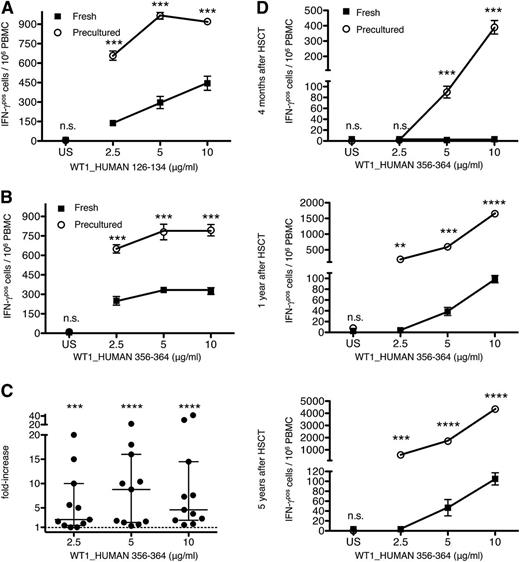
The enhanced sensitivity of CD8 T cells from HD precultured PBMCs to multiple virus-specific antigens, relative to CD8 T cells from fresh PBMCs, encouraged us to test the effect of this protocol on the detection of CD8 T-cell responses directed against tumors. We selected peptide antigens derived from the human protein WT1, which is highly expressed in several forms of leukemia that are treated by myeloablation and allogeneic HSCT.23-25 Consistent with published results, we found that the frequency of IFN-γ secreting T cells specific for peptides derived from the tumor-associated antigen WT1_HUMAN was often low compared with high-avidity CD8 T-cell responses to HCMV, EBV, or influenza A (Figure 5A-B).26,27 As shown in Figure 5A-B, restored T cells from representative HLA–A0201-positive patients displayed dramatically higher IFN-γ recall responses to titrated amounts of the peptides WT1_HUMAN 126-134 and 356-364 than T cells from fresh PBMCs. In fact, the ratio of precultured to fresh PBMC responses following stimulation with WT1_HUMAN 356-364 (Figure 5C) was consistently even higher than the gain observed upon stimulation with the viral peptide pool (Figure 3E).
HD preculture enhances the recall responses of PBMCs to WT1-derived antigens. (A-B) IFN-γ T-cell responses from fresh or precultured PBMCs of 1 representative HLA–A0201-positive HSCT patient to titrated amounts of the peptide WT1_HUMAN 126-134 or WT1_HUMAN 356-364, respectively. Unpaired Student t test: ***P < .0005. Data represent mean ± SD for triplicate samples. The experiment presented in (A) was repeated for 2 different patients. (C) Compiled data from Figure 4B from 11 patients. Average time between stem cell transplantation and sampling: 814 days; from 166 to 2372 days. Data are presented as in Figure 2B. Raw data: supplemental Figure 5D. Mann-Whitney U test: ***P < .0005; ****P < .0001. Data represent median with IQR. (D) Improved monitoring of anti–WT1-directed T-cell responses from an HLA–A0201-positive patient 4 months (top), 1 year (middle), and 5 years (bottom) after HSCT upon HD preculture of PBMCs. Unpaired Student t test: **P < .005; ***P < .0005; ****P < .0001. Data represent mean ± SD for triplicate samples. n.s., not significant.
HD preculture enhances the recall responses of PBMCs to WT1-derived antigens. (A-B) IFN-γ T-cell responses from fresh or precultured PBMCs of 1 representative HLA–A0201-positive HSCT patient to titrated amounts of the peptide WT1_HUMAN 126-134 or WT1_HUMAN 356-364, respectively. Unpaired Student t test: ***P < .0005. Data represent mean ± SD for triplicate samples. The experiment presented in (A) was repeated for 2 different patients. (C) Compiled data from Figure 4B from 11 patients. Average time between stem cell transplantation and sampling: 814 days; from 166 to 2372 days. Data are presented as in Figure 2B. Raw data: supplemental Figure 5D. Mann-Whitney U test: ***P < .0005; ****P < .0001. Data represent median with IQR. (D) Improved monitoring of anti–WT1-directed T-cell responses from an HLA–A0201-positive patient 4 months (top), 1 year (middle), and 5 years (bottom) after HSCT upon HD preculture of PBMCs. Unpaired Student t test: **P < .005; ***P < .0005; ****P < .0001. Data represent mean ± SD for triplicate samples. n.s., not significant.
To demonstrate the specificity of the HLA-A0201–restricted WT1 responses, PBMCs of the HIV sero-negative patients presented in Figure 5A-B were also treated with the HLA-A0201–restricted peptide HIV-1 pol 476-484 (supplemental Figure 3A-B), and showed no response. Nonspecific effects of WT1 peptides were furthermore excluded by the unresponsiveness of PBMCs from an HLA-A0201 negative donor with WT1_HUMAN 126-134 and 356-364 (supplemental Figure 3C).
To test the usefulness of the RESTORE protocol for monitoring the antitumor response of leukemia patients who underwent HSCT, we tested WT1-specific CD8 T-cell responses with or without HD preculture of PBMCs at different time points after transplantation (Figure 5D). Antitumor responses were detected already 4 months after HSCT, if the RESTORE protocol and not the conventional assay was used. Furthermore, we observed dramatically higher WT1 responses in HD precultured PBMCs compared with freshly thawed PBMCs as late as after 5 years of transplantation.
HD preculture allows the generation of CD8 T-cell lines with an improved representation of clones responding to low antigen concentrations
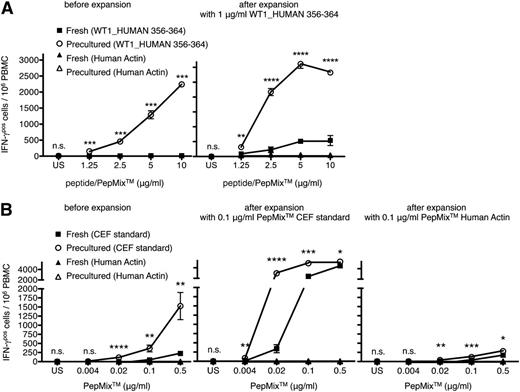
Repeated antigen stimulation in the presence of growth-promoting cytokines is often used to generate T-cell lines and clones for both analytical and therapeutic purposes. The gain in antigen-sensitivity during the first round of in vitro stimulation after HD preculture suggested that without this step, CD8 T cells able to respond to low antigen concentrations if appropriately presensitized by cell contacts may be lost from the clonally expanded population, and hence be under-represented during later rounds of stimulation. Recall responses of fresh or precultured PBMCs to titrated amounts of the peptide WT1_HUMAN 356-364 were analyzed before and after antigen-specific CD8 T-cell expansion in the presence of the cytokines IL-2, -7, and -15 (Figure 6A). In agreement with our above results, functional WT1-specific CD8 T-cell responses were absent or barely measurable in fresh PBMCs of patients, whereas CD8 T-cell responses from precultured PBMCs were easily detectable (Figure 6A, left). Importantly, the enhancing effect of HD preculture before the first round of stimulation with WT1_HUMAN 356-364 was apparent even after long-term culture. As shown for 1 representative patient (Figure 6A, right) CD8 T cells from expanded precultured PBMCs displayed a much higher sensitivity to WT1_HUMAN 356-364 in comparison with CD8 T cells expanded from fresh PBMCs that were cultured under the same conditions. Of note, the increase in the CD8 T-cell response was particularly striking at low antigen doses, suggesting that indeed, the HD preculture step had allowed presensitization-dependent clones to participate in the antigen-driven expansion. No responses were observed to actin-derived peptides, illustrating the maintenance of specificity for the peptides employed for expansion.
HD preculture of PBMCs allows the generation of CD8 T-cell lines with an improved representation of clones responding to low antigen concentrations. (A, left) IFN-γ responses of CD8 T cells from fresh or precultured PBMCs of 1 HLA–A0201-positive patient to titrated amounts of the peptide WT1_HUMAN 356-364 before expansion. (Right) Fresh or precultured PBMCs of the same patient were stimulated with 1 μg/ml of the peptide WT1_HUMAN 356-364 in a final cell density of 2 × 106 cells/ml and were expanded in the presence of IL-2, IL-7, and IL-15 for 2 weeks. After expansion, cells were re-stimulated with titrated amounts of the peptide WT1_HUMAN 356-364 or the control pool human actin in IFN-γ ELISPOT plates for 16 hours. Unpaired Student t test: **P < .005; ***P < .0005; ****P < .0001. (B, left) IFN-γ responses of CD8 T cells from fresh or precultured PBMCs of 1 representative healthy donor to titrated amounts of the HLA-class I restricted PepMix CEF standard and the control pool human actin before expansion. Fresh or precultured PBMCs of the same donor were stimulated with 0.1 μg/ml of the PepMix CEF standard (middle) or the PepMix Human Actin (right), and were expanded under the same conditions as presented in (A). After expansion, virus-specific CD8 T cells were re-stimulated with titrated amounts of the PepMix CEF standard or the control pool human actin in IFN-γ ELISPOT plates for 16 hours, respectively. Unpaired Student t test: *P < .05; **P < .005; ***P < .0005; ****P < .0001. Data represent mean ± SD for triplicate samples. Experiments were repeated 4 times.
HD preculture of PBMCs allows the generation of CD8 T-cell lines with an improved representation of clones responding to low antigen concentrations. (A, left) IFN-γ responses of CD8 T cells from fresh or precultured PBMCs of 1 HLA–A0201-positive patient to titrated amounts of the peptide WT1_HUMAN 356-364 before expansion. (Right) Fresh or precultured PBMCs of the same patient were stimulated with 1 μg/ml of the peptide WT1_HUMAN 356-364 in a final cell density of 2 × 106 cells/ml and were expanded in the presence of IL-2, IL-7, and IL-15 for 2 weeks. After expansion, cells were re-stimulated with titrated amounts of the peptide WT1_HUMAN 356-364 or the control pool human actin in IFN-γ ELISPOT plates for 16 hours. Unpaired Student t test: **P < .005; ***P < .0005; ****P < .0001. (B, left) IFN-γ responses of CD8 T cells from fresh or precultured PBMCs of 1 representative healthy donor to titrated amounts of the HLA-class I restricted PepMix CEF standard and the control pool human actin before expansion. Fresh or precultured PBMCs of the same donor were stimulated with 0.1 μg/ml of the PepMix CEF standard (middle) or the PepMix Human Actin (right), and were expanded under the same conditions as presented in (A). After expansion, virus-specific CD8 T cells were re-stimulated with titrated amounts of the PepMix CEF standard or the control pool human actin in IFN-γ ELISPOT plates for 16 hours, respectively. Unpaired Student t test: *P < .05; **P < .005; ***P < .0005; ****P < .0001. Data represent mean ± SD for triplicate samples. Experiments were repeated 4 times.
An analogous set of experiments was performed regarding the generation of virus-specific CD8 T-cell lines. CD8 T cells from precultured PBMCs showed a higher IFN-γ recall response to titrated amounts of the PepMix CEF standard compared with CD8 T cells from fresh PBMCs (Figure 6B, left). Even after expansion of virus-specific CD8 T cells during long-term culture, the enhanced responsiveness of CD8 T cells expanded from precultured PBMCs was maintained if cells were re-stimulated with low concentrations of peptides (0.004 to 0.1 μg/mL; Figure 6B, middle). Of note, this enhanced response of cells expanded from HD precultured PBMCs remained strictly antigen-dependent. Furthermore, expansion and re-stimulation with peptides derived from human actin yielded only minute responses to this self-antigen using both protocols (Figure 6B, right).
Phosphorylation of proximal TCR signaling components in HD preculture-derived CD8 T cells
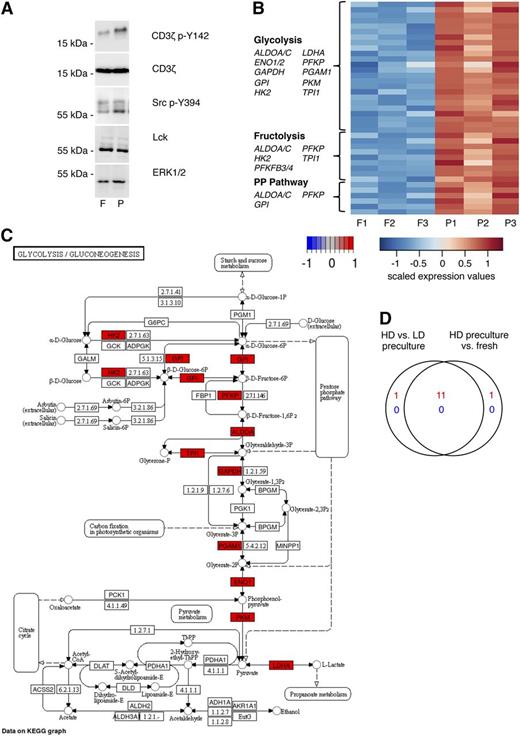
In mice, MHC scanning in the tissue context results in phosphorylation of proximal compounds of the signaling machinery.9 This can be detected by western blotting using CD3ζ p-Y142–specific mAb, which recognize a product with an apparent shift in electrophoretic mobility from 16 to 21 kDa. Indeed, CD3ζ p-Y142 was detected at a higher level in HD preculture-derived compared with freshly isolated CD8 T cells (Figure 7A). In addition, Lck p-Y394, which is required for tyrosine phosphorylation of the CD3ζ chains,28,29 was more pronounced in CD8 T cells from precultured PBMCs compared with CD8 T cells from fresh PBMCs.
HD preculture of PBMCs affects TCR signaling status and metabolism of CD8 T cells. (A) Phosphorylation of proximal TCR signaling components were compared between isolated CD8 T cells from fresh and precultured PBMCs by western blotting. Membranes were probed with antibodies against CD3ζ p-Y142, CD3ζ, Src p-Y394, and Lck. ERK1/2 served as a loading control. (B) Differentially expressed probe sets assigned to the metabolic pathways, glycolysis, fructolysis, and pentose phosphate pathway from a comparison of CD8+CD45R0+ T cells from fresh and HD-precultured PBMCs are shown in a heatmap of scaled expression values. (C) Differentially expressed glycolysis pathway genes of the same experiment are shown in a KEGG pathway map with color-coded log-fold changes. (D) High concordance of changes in glycolysis-related gene expression changes between CD8+CD45R0+ T cells from fresh PBMCs (presented in B-C) and pan T cells from LD precultured PBMCs as shown by Venn diagram display. F, fresh; HD, high density; LD, low density; P, precultured.
HD preculture of PBMCs affects TCR signaling status and metabolism of CD8 T cells. (A) Phosphorylation of proximal TCR signaling components were compared between isolated CD8 T cells from fresh and precultured PBMCs by western blotting. Membranes were probed with antibodies against CD3ζ p-Y142, CD3ζ, Src p-Y394, and Lck. ERK1/2 served as a loading control. (B) Differentially expressed probe sets assigned to the metabolic pathways, glycolysis, fructolysis, and pentose phosphate pathway from a comparison of CD8+CD45R0+ T cells from fresh and HD-precultured PBMCs are shown in a heatmap of scaled expression values. (C) Differentially expressed glycolysis pathway genes of the same experiment are shown in a KEGG pathway map with color-coded log-fold changes. (D) High concordance of changes in glycolysis-related gene expression changes between CD8+CD45R0+ T cells from fresh PBMCs (presented in B-C) and pan T cells from LD precultured PBMCs as shown by Venn diagram display. F, fresh; HD, high density; LD, low density; P, precultured.
Metabolic reprogramming in CD8 memory T cells upon HD preculture of PBMCs
Finally, we performed gene expression analysis of CD8 memory T cells from fresh and HD precultured PBMCs to determine functional clusters that could additionally contribute to the enhanced functionality of HD precultured CD8 T cells. Interestingly, multiple genes related to metabolism were differentially expressed (Figure 7B). Whereas genes involved in glycolysis (33 probe sets, 12 genes), fructolysis (9 probe sets, 7 genes), and the pentose phosphate pathway (6 probe sets, 4 genes) were upregulated, expression levels of genes related to lipolysis and amino acid synthesis remained unchanged. Thus, CD8 memory T cells in HD precultures apparently reprogram their metabolism in preparation for antigenic stimulation, similar to cancer cells,30 effector T cells,31-33 and activated macrophages34 that switch from oxidative phosphorylation to aerobic glycolysis in order to facilitate uptake and incorporation of nutrients into macromolecules (Figure 7C).32,35,36 Of note, the key glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase is also a positive translational regulator of IFN-γ production.31,32
To exclude that the enhanced expression of glycolytic genes in CD8 memory T cells is a result of in vitro culture per se, rather than of the HD preculture conditions that lead to increased antigen sensitivity, we compared our results with a previous data set (Gene Expression Omnibus database, accession number GSE51288) in which CD4 and CD8 T cells were isolated from either high- or low-density precultured PBMCs (Figure 7D). A large overlap of 11/13 genes associated with the functional cluster “glycolysis” demonstrates that metabolic reprogramming of T cells is induced specifically by tissue-like conditions provided by HD.
Discussion
In this study, we report that returning circulating CD8 T cells to tissue-like conditions by first preculturing PBMCs at HD (the RESTORE protocol that was initially described for the CD4 T-cell response to TGN141211 ), results in a dramatic increase in their responsiveness to antigens.
The increase in the magnitude of CD8 T-cell responses obtained by preculturing PBMCs is a robust phenomenon. Thus, the frequency of IFN-γ–producing CD8 T cells responding to viral antigens increased by twofold on average (from 1.2- to eightfold) if the whole range of antigen doses is considered (Figure 3E). Remarkably high (sometimes >10-fold) increases in sensitivity (ie, reduction in the antigen concentration required to obtain the same response as in standard PBMC cultures) were detected after stimulation with titrated amounts of defined virus-derived peptides (Figure 2A,C), as well as virus-derived peptide pools, leading to saturated T-cell responses to multiple virus-specific peptides with as little as 0.3 μg/mL (Figure 3A). In contrast, even after applying the RESTORE protocol, much higher peptide concentrations (5 to 10 μg/mL) were necessary to trigger saturated T-cell responses to tumor-directed peptides (Figure 5A-B). This corroborates with reports indicating a higher functional avidity of T cells for APCs displaying virus-specific antigens compared with over-expressed autologous tumor-derived antigens.26,27
The importance of preceding cell-cell interactions for the responsiveness of CD8 T cells to “weak” tumor-associated antigens was unambiguously demonstrated by the dramatic improvement of PBMC responses to WT1_HUMAN 356-364 after HD preculture (Figure 5C). Here, the gain in sensitivity was such that it allowed the detection of tumor-directed CD8 T-cell responses in some patients that were undetectable in standard assays performed with fresh PBMCs (Figure 6A). High fold-increases in the magnitude of precultured relative to fresh PBMC responses (up to fourfold) were also observed for T cells specific for WT1_HUMAN 126-134 of 2 leukemia patients expressing the allele A0201 (data not shown).
The functional impairment of CD4 T cells during their sojourn in the blood has been clearly demonstrated in both mice9 and humans,11 and is associated with a reduction in the phosphorylation and polarization of signaling molecules, which can be restored by an optimized HD preculture.11 With regard to the mechanisms underlying the increase in sensitivity of restored CD8 T cells, we extended the observations made for CD4 T cells with regard to preactivation of the TCR signaling machinery11 to tyrosine phosphorylation of Lck and the TCRζ chain (Figure 7A).
Given the established need for T cells to reprogram their metabolism in response to activation,31,33,35,36 we compared KEGG pathways in CD8 memory T cells from HD precultured PBMCs compared with fresh PBMCs, and found an upregulation of metabolic enzymes that are shared by glycolysis, fructolysis, and the pentose phosphate pathway (Figure 7B-D).
The importance of continuous cellular interactions provided by the tissue environment for the maintenance of full T-cell reactivity has been demonstrated for murine9 and human11 lymph nodes where short-term suspension culture leads to a reduction in CD4 T-cell responsiveness. Here, we extended these findings to the antiviral CD8 T-cell response of the tonsil by demonstrating a fivefold to 10-fold drop in antigen-sensitivity within 2 hours of dispersed culture at body temperature (Figure 4A).
Importantly, returning PBMC T cells to tissue-like conditions before antigenic stimulation does not involve antigen- or cytokine-driven clonal expansion, as is used in some protocols to reveal otherwise undetectable responses.22 Indeed, HLA-A0201/pp65 pentamer staining (a procedure unsuitable for routine clinical practice due to the multitude of pentamers required) reveals that the frequency of CD8 T cells expressing the relevant antigen/MHC specificity is unaffected by the preculturing step (supplemental Figure 4). This feature makes HD preculture an ideal and simple tool for immunomonitoring of CD8 T-cell responses to cancer-associated antigens over time (Figure 5D), and to infectious agents and vaccines. Finally, it shall be mentioned that resetting of circulating T cells to a tissue-like state before the first in vitro contact with antigen, avoids the loss of those antigen-specific cells that depend on presensitization for an antigen-specific response. The inclusion of these cells also in long-term expanded CD8 T-cell lines and clones may improve their usefulness for analytic and therapeutic purposes by harnessing cytotoxic T-lymphocytes that could contribute to the effectiveness of passive cellular vaccination against infectious agents and tumors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Margarete Göbel, Elke Baumeister, Hemant Joshi, and Susanne Berr for excellent technical assistance.
This study was supported by the Vogel Foundation, Würzburg (Forschungsförderpreis 2014), a grant of the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg (J.W.), and the IZKF Würzburg (Project Z-6) (C.J.S.).
Authorship
Contribution: J.W. performed all presented experiments with the exception of the microarray hybridization; C.J.S. analyzed microarray data; S.H. and G.U.G. provided blood and tissue material; S.C., D.T., and A.A.M. provided reagents and discussed the data; and T.H. directed the study and wrote the report with J.W. with input from S.S., C.-J.S., S.C., D.T., and A.A.M.
Conflict-of-interest disclosure: S.C., D.T., and A.A.M. are employees of TheraMAB LLC and T.H. is a consultant to TheraMAB LLC. The remaining authors declare no competing financial interests.
Correspondence: Thomas Hünig, Institute of Virology and Immunobiology, University of Würzburg, Versbacher Strasse 7, 97078 Würzburg, Germany; e-mail: huenig@vim.uni-wuerzburg.de.