Abstract
Hodgkin lymphoma (HL) is a highly curable form of childhood cancer, with estimated 5 year survival rates exceeding 98%. However, the establishment of a “standard of care” approach to its management is complicated by the recognition that long-term overall survival declines in part from delayed effects of therapy and that there continue to be subgroups of patients at risk for relapse for which prognostic criteria cannot adequately define. This challenge has resulted in the development of various strategies aimed at identifying the optimal balance between maintaining overall survival and avoidance of long-term morbidity of therapy, often representing strategies quite different from those used for adults with HL. More precise risk stratification and methods for assessing the chemosensitivity of HL through imaging studies and biomarkers are in evolution. Recent advances in the understanding of the biology of HL have led to the introduction of targeted therapies in both the frontline and relapsed settings. However, significant barriers exist in the development of new combination therapies, necessitating collaborative studies across pediatric HL research consortia and in conjunction with adult groups for the adolescent and young adult (AYA) population with HL.
Hodgkin lymphoma (HL) is one of the most curable forms of childhood cancer, with estimated 5 year survival rates exceeding 98%,1 yet long-term overall survival continues to decline, from both delayed deaths from HL and late effects of therapy.2 Given its earlier age of presentation compared with many more prevalent adult cancers, the impact of premature mortality in children and adults with HL carries the second highest societal cost due to economic loss of productivity.3 Thus, the major focus in pediatric HL is the development of various strategies aimed at identifying the optimal balance between maintaining high rates of overall survival and avoiding the long-term morbidity and mortality associated with HL directed therapy, with strategies that are often quite different than those used for adults with HL.
With increasing understanding of the unique pathology of HL and the identification of novel targeted agents, there is an opportunity to further refine the therapy in order to improve the therapeutic index to maximize cure and reduce late toxicities of therapy. In this review, a summary of recent advances is presented, along with a discussion of opportunities for study specific to the pediatric HL setting.
Lessons from recent clinical trials in children and adolescents with Hodgkin lymphoma
Recent clinical trials in children and adolescents have shifted away from the ABVD regimens more common in adults with HL by utilizing different combinations of conventional chemotherapy, and by investigating the role of early response to chemotherapy for subsequent therapeutic stratification, particularly in regard to the delivery of radiation therapy (RT; Tables 1 and 2).4-13 The German Society of Pediatric Oncology and Hematology (GPOH) and now in an European pediatric and adolescent Hodgkin lymphoma network (Euronet-HD) has investigated OEPA (vincristine, etoposide, prednisone, doxorubicin) for low-risk, and OEPA with COPDac (cyclophosphamide, vincristine, prednisone, dacarbazine) for intermediate and high-risk groups.7 In North America, the Children's Oncology Group has primarily evaluated ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide) and its derivatives across the risk groups.5,6,11 Advantages of these regimens include lower cumulative doses of anthracyclines, alkylating agents, and bleomycin compared with the MOPP and ABVD regimens and their variations used in prior decades, which is expected to translate into a reduction in second malignant neoplasms, cardiovascular, pulmonary, and fertility complications in the long-term.
Recent clinical trials for pediatric low-risk Hodgkin lymphoma
| Study . | N . | Risk group . | Rx . | Radiation (Gy) . | Directly assigned to radiation, % . | EFS or DFS, %; OS, % (y) . |
|---|---|---|---|---|---|---|
| Children’s Oncology Group | ||||||
| CCG59424 | 215 | IA, IB, IIA without adverse features* | COPP/ABV ×4 | CR after cycle 4: randomized to 21, IF | 23% of overall study population (not restricted to low risk) | IF: 97.1 None: 89.1 (p = .001); |
| POG94265 | 294 | IA, IIA, IIIA (no bulk) | DBVE ×2-4 (based on response after cycle 2) | vs none; PR: 21, IF 25.5, IF | 100 | IF: 100 None: 95.9 (p = 0.5) (10) 86.2; 97.4 (8) |
| AHOD04316 | 287 | IA, IIA (no bulk) | AVPC ×3 | CR after cycle 3: none PR: 21, IF | 37 | 79.8; 99.6 (4) |
| German Society of Pediatric Oncology | ||||||
| GPOH7 | 195 | IA, IB, IIA | OEPA (males) | CR after cycle 2: no RT; PR after cycle 2: 20-30, IF | 70 | 92; 99.5 (5) |
| OPPA (females) ×2 | ||||||
| French Society of Pediatric Oncology (SFOP) | ||||||
| MDH-908 | 202 | IA, IB, IIA, IIB | VBVP ×4 (*OPPA ×1-2 if PR after cycle 4) | 20-40, IF | 100 | 91.1, 97.5 (5) |
| Stanford, Dana Farber and St Jude Consortium | ||||||
| Stanford, Dana Farber, and St Jude Consortium9 | 110 | IA, IB, IIA, IIB no bulk, no E | VAMP ×4 | 15-22.5, IF | 100 | 89.4; 96.1 (10) |
| Stanford, Dana Farber, and St Jude Consortium17 | 88 | IA, IIA, <3 nodal sites, no bulk, no E | VAMP ×4 | CR after 2 cycles: no RT; PR after cycle 2: 25.5 IF | 47 | CR: 89.4; PR: 92.5 (2) |
| Study . | N . | Risk group . | Rx . | Radiation (Gy) . | Directly assigned to radiation, % . | EFS or DFS, %; OS, % (y) . |
|---|---|---|---|---|---|---|
| Children’s Oncology Group | ||||||
| CCG59424 | 215 | IA, IB, IIA without adverse features* | COPP/ABV ×4 | CR after cycle 4: randomized to 21, IF | 23% of overall study population (not restricted to low risk) | IF: 97.1 None: 89.1 (p = .001); |
| POG94265 | 294 | IA, IIA, IIIA (no bulk) | DBVE ×2-4 (based on response after cycle 2) | vs none; PR: 21, IF 25.5, IF | 100 | IF: 100 None: 95.9 (p = 0.5) (10) 86.2; 97.4 (8) |
| AHOD04316 | 287 | IA, IIA (no bulk) | AVPC ×3 | CR after cycle 3: none PR: 21, IF | 37 | 79.8; 99.6 (4) |
| German Society of Pediatric Oncology | ||||||
| GPOH7 | 195 | IA, IB, IIA | OEPA (males) | CR after cycle 2: no RT; PR after cycle 2: 20-30, IF | 70 | 92; 99.5 (5) |
| OPPA (females) ×2 | ||||||
| French Society of Pediatric Oncology (SFOP) | ||||||
| MDH-908 | 202 | IA, IB, IIA, IIB | VBVP ×4 (*OPPA ×1-2 if PR after cycle 4) | 20-40, IF | 100 | 91.1, 97.5 (5) |
| Stanford, Dana Farber and St Jude Consortium | ||||||
| Stanford, Dana Farber, and St Jude Consortium9 | 110 | IA, IB, IIA, IIB no bulk, no E | VAMP ×4 | 15-22.5, IF | 100 | 89.4; 96.1 (10) |
| Stanford, Dana Farber, and St Jude Consortium17 | 88 | IA, IIA, <3 nodal sites, no bulk, no E | VAMP ×4 | CR after 2 cycles: no RT; PR after cycle 2: 25.5 IF | 47 | CR: 89.4; PR: 92.5 (2) |
VBVP indicates vinblastine, bleomycin, etoposide, prednisone; VAMP, vinblastine, doxorubicin, methotrexate, prednisone; COPP/ABV, cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine; DBVE, doxorubicin, bleomycin, vincristine and etoposide; OEPA, vincristine, etoposide, prednisone, doxorubicin; OPPA, vincristine, prednisone, procarbazine, doxorubicin; ABVE-PC, doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, procarbazine; DECA, dexamethasone, etoposide, cisplatin, cytarabine; SFOP, French Pediatric Oncology Society; CCG, Children’s Cancer Study Group; COG, Children’s Oncology Group; GPOH, German Society of Pediatric Oncology and Hematology; POG, Pediatric Oncology Group; IF, involved field; RER, rapid early responder; and SER, slow early responder.
Adverse features: hilar disease, bulk, ≥4 nodal regions, mediastinal bulk.
Recent clinical trials for pediatric intermediate and high-risk Hodgkin lymphoma
| Study . | N . | Risk group . | Rx . | Radiation (Gy) . | Directly Assigned to Radiation, % . | EFS, % (y) . |
|---|---|---|---|---|---|---|
| Children’s Oncology Group | ||||||
| CCG59424 | Intermediate: 394 | Intermediate: IA, IB, IIA with adverse features; IIB, III | Intermediate: COPP/ABV ×6 | CR after cycle 6: randomized to 21, IF | 23% of overall study population (not restricted to low risk) | Intermediate: |
| High: 141 | High: IV | High: COPP/ABV, CHOP, etoposide/cytarabine ×2 | vs none; PR: 21, IF | RT: 87; 95 No RT: 83; 100 High: RT: 90; 100 No RT: 81; 94 (10) | ||
| POG942511 | Intermediate: 53 | Intermediate: IB, IIA bulk, IIIA bulk | DBVE-PC ×3-5 (based on response after cycle 3) | 25.5, IF | 100% | Intermediate: 84%; OS NR |
| High: 163 | High: IIB, IIIB, IV | High: 85; OS NR (5) | ||||
| CCG5970412 | 98 | IIB/IIIB with bulk, IV | BEACOPP ×4, RER F: COPP/ABV ×4 RER M: ABVD ×2 SER: BEACOPP ×4 | RER F: None RER M 21, IF SER: 21, IF | 61% | 94; 97 (5) |
| AHOD003127 | 1734 | IB, IA/IIA with bulk, IIB, IIIA, IVA | ABVE-PC ×4, | RER, CR: None | 53% | Overall: |
| SER: ±DECA ×2 | RER, <CR: 21, IF SER: 21, IF | 85; 97.8 RER: 86.9; 98.5 SER: 77.4; 95.3 (4) | ||||
| German Society of Pediatric Oncology | ||||||
| GPOH7 | Intermediate: 139 | Intermediate: IEA/B, IIEA, IIB, IIIA | OPPA (female); OEPA (male) ×2 | 19.8-35 IF | 100% | Intermediate: 88.3; 99.5 |
| High: 239 | High: IIEB, IIIEA/B, IIIB, IV | Intermediate: COPDAC ×2 | High: 86.9; 94.9 | |||
| High: COPDAC ×4 | (5) |
| Study . | N . | Risk group . | Rx . | Radiation (Gy) . | Directly Assigned to Radiation, % . | EFS, % (y) . |
|---|---|---|---|---|---|---|
| Children’s Oncology Group | ||||||
| CCG59424 | Intermediate: 394 | Intermediate: IA, IB, IIA with adverse features; IIB, III | Intermediate: COPP/ABV ×6 | CR after cycle 6: randomized to 21, IF | 23% of overall study population (not restricted to low risk) | Intermediate: |
| High: 141 | High: IV | High: COPP/ABV, CHOP, etoposide/cytarabine ×2 | vs none; PR: 21, IF | RT: 87; 95 No RT: 83; 100 High: RT: 90; 100 No RT: 81; 94 (10) | ||
| POG942511 | Intermediate: 53 | Intermediate: IB, IIA bulk, IIIA bulk | DBVE-PC ×3-5 (based on response after cycle 3) | 25.5, IF | 100% | Intermediate: 84%; OS NR |
| High: 163 | High: IIB, IIIB, IV | High: 85; OS NR (5) | ||||
| CCG5970412 | 98 | IIB/IIIB with bulk, IV | BEACOPP ×4, RER F: COPP/ABV ×4 RER M: ABVD ×2 SER: BEACOPP ×4 | RER F: None RER M 21, IF SER: 21, IF | 61% | 94; 97 (5) |
| AHOD003127 | 1734 | IB, IA/IIA with bulk, IIB, IIIA, IVA | ABVE-PC ×4, | RER, CR: None | 53% | Overall: |
| SER: ±DECA ×2 | RER, <CR: 21, IF SER: 21, IF | 85; 97.8 RER: 86.9; 98.5 SER: 77.4; 95.3 (4) | ||||
| German Society of Pediatric Oncology | ||||||
| GPOH7 | Intermediate: 139 | Intermediate: IEA/B, IIEA, IIB, IIIA | OPPA (female); OEPA (male) ×2 | 19.8-35 IF | 100% | Intermediate: 88.3; 99.5 |
| High: 239 | High: IIEB, IIIEA/B, IIIB, IV | Intermediate: COPDAC ×2 | High: 86.9; 94.9 | |||
| High: COPDAC ×4 | (5) |
Abbreviations are explained in Table 1.
Radiotherapy usage varies considerably as indicated in Tables 1 and 2. The Children's Oncology Group recently reported that RT may be safely omitted in intermediate risk patients (including 73% with bulk disease at diagnosis) who have a rapid reduction in tumor dimensions by CT after 2 cycles of chemotherapy, resulting in half of intermediate risk patients showing no significant benefit with adjuvant RT.13 The GPOH has omitted RT for the 30% of low-risk patients achieving a CR after 2 cycles of OEPA, whereas intermediate and high-risk patients have continued to receive RT irrespective of response.7 In general, pediatric radiotherapy approaches utilize lower doses (15-25Gy) and fields (involved field or node).14 The impact of improvements in radiotherapy planning with PET and CT guided planning and the restriction of radiation to slow responding sites of disease is being evaluated in a recently completed COG trial (NCT01026220). Furthermore, the use of proton therapy is being assessed in a COG trial for high-risk HL (NCT02166463), which may allow further reductions in normal tissue exposure by minimizing the exit dose.
No prospective comparisons of efficacy or toxicity for pediatric versus adult regimens for adolescents and young adults has been reported to date. A retrospective study including 245 adolescent patients with HL aged 14-21 who were treated with either pediatric or adult protocols demonstrated superior EFS rates for adolescents <18 years treated with pediatric regimens.15 The German Hodgkin Study Group is testing a new combination (NCT 01569204) in which dacarbazine is substituted for procarbazine, inspired in part from the GPOH experience with COPDac. Collaborations are currently underway between the COG and the North American cooperative research groups for the development of common treatment protocols for select groups of adolescent and young adults (AYA) with HL.
For patients with relapsed or primary refractory disease, the potential for cure remains ∼50% with current therapies including high-dose chemotherapy and autologous hematopoietic stem cell transplantation (AHSCT). A recent CIBMTR analysis of 671 patients <30 years who underwent AHSCT for relapsed/refractory HL reported progression free survival and overall survival rates of 56% and 73%, respectively.16 Clinical risk factors including first remission <12 months, poor performance status, chemoresistance, extranodal disease at relapse, and receipt of regimens other than ABVD/ABVD-like for first line therapy predict reduced survival post AHSCT. In contrast, patients with late relapse of low-stage disease are predicted to have excellent outcomes with conventional chemotherapy or chemoradiotherapy, and thus may be able to avoid the acute and long-term toxicity associated with AHSCT.17
Priorities for clinical research
At diagnosis, patients are classified into risk groups based on stage and the presence of clinical, biologic, and serologic risk factors. Prognostic factors are used to assign patients to categories for risk-adapted therapy. In adults with HL, the International Prognostic Score (IPS) was developed as an effort to better predict patients with a poor prognosis who might benefit from therapy intensification. However, there is no uniform system of prognostic stratification in pediatric HL and different clinical trial groups have established different risk categories, thus making comparison across studies challenging. Retrospective studies in pediatric HL have identified clinical prognostic factors but these have not been validated across various treatment regimens,18 although prospective validation of the Childhood Hodgkin International Prognostic Score19 is ongoing. Refinement of risk stratification will be gained through the incorporation of correlative biomarkers of the tumor and host; however, to date none have demonstrated significant clinical benefit, especially in the pediatric HL setting.
Early response to chemotherapy may direct omission of radiation therapy but the optimal method of evaluating response has not been defined. In contrast to recent adult trials, reported pediatric trials by COG and European have defined early response and complete remission using computed tomography (CT) based on reductions in either 2-dimensional area or volume. Inter-observer variability in assessing tumor reductions and the lack of data on the optimal threshold has led to difficulties in implementing these definitions in clinical practice. Fluorine-18 2-fluoro-2-deoxy-D-glucose–positron emission tomography (FDG-PET) has become an established technique for initial staging of HL and may have prognostic value during treatment by detecting metabolic activity to distinguish between residual disease and necrosis or fibrosis. It is important to emphasize that interim PET/CT has not been validated as a predictive endpoint and its use in this setting remains investigational. Recent COG and Euronet trials have used FDG-PET criteria for assessing response and in some studies, results have directed further therapy. Normalization of PET uptake after one cycle of chemotherapy using visual criteria is prognostic in low-risk HL.6 The current COG high-risk trial is prospectively investigating the predictive value of the semiquantitative 5-point Deauville criteria20 for RT determination whereas the Euronet is investigating a similar method (qPET) in the context of the recently completed C1 trial.21 Final results of these strategies are still awaited from recently completed trials from both COG and Euronet in other HL subgroups. In the interim, an international effort to harmonize staging and response criteria is underway.
Novel approaches for patients who relapse after initial therapy or following AHSCT are needed; however, the small numbers of patients limit the opportunity to evaluate these approaches in pediatric specific trials. With the observation that complete response by PET/CT is associated with higher survival following AHSCT,16 evaluation of novel combinations to improve CR rates would be of value, perhaps in conjunction with adult cooperative groups as part of an AYA initiative. Given that the median age at relapse is in mid adolescence, consideration for lowering the age for eligibility for trials would greatly improve access to novel agents for adolescents with HL.
Recent insights into the biology of Hodgkin lymphoma
HL is characterized by the presence of multinucleated giant cells (Hodgkin/Reed-Sternberg cells; H/RS) or large mononuclear cell variants (lymphocytic and histiocytic cells) accounting for ∼1% of the cells in a background of inflammatory cells consisting of small lymphocytes, histiocytes, neutrophils, eosinophils, plasma cells, and fibroblasts. Scientists have long been puzzled by why the very fragile H/RS cell rarely grows in cell culture, yet in vivo, the cell survives despite being surrounded by immunoreactive cells. New insights into the biology of HL are beginning to provide an understanding of its pathogenesis.
H/RS cells manipulate their microenvironment by suppressing antitumor immune surveillance through a variety of mechanisms (for review, see Diefenbach and Steidl22 ). The H/RS cells secrete cytokines, such as TARC (CCL17), CCL5, and CCL22 attracting T-helper 2 and regulatory T (Treg) cells to the tumor microenvironment, and interleukin-7, which induces differentiation of naïve CD4+ T cells toward FoxP3+ Treg cells. NF-κB is constitutively expressed by H/RS, in part as a result of somatic mutations in pathway members and regulators, as well as other antiapoptotic proteins that inhibit the intrinsic and extrinsic apoptotic pathways. H/RS cell surface molecule overexpression maintains tolerance, such as galectin-1, which is correlated with decreased infiltration of CD8+ effector cells at the tumor site and Fas ligand, which induces apoptosis in tumor-specific cytotoxic T lymphocytes. Upregulation of the programmed death ligand PD-L1 on H/RS cells induces anergy in peritumoral T cells. Moreover, chromosomal rearrangements of the master regulator of MHC class II expression, CIITA, result in downregulated major histocompatibility complex (MHC) class II expression and overexpression of fusion partners such as PD-L1 and PD-L2. Thus, T-cell exhaustion and deficient antitumor immunity enable progression of HL.
At the present time there are no validated integral biomarkers that can be utilized for risk stratification or as surrogate markers of outcome. Whereas a wide range of biomarkers providing information on H/RS cells, the microenvironment and host polymorphisms and mutations,23 have been studied in adults with HL, limited information is available specifically in pediatric HL. Comprehensive molecular characterization of childhood HL may help to further refine disease classification and improve our assessment of early response. Among the more promising biomarkers, gene expression profiling from paraffin-embedded tissue in adult HL has identified a 23-gene expression classifier from the E2496 Intergroup Trial.24 A collaborative study is underway to determine if the adult HL expression signature can accurately predict outcomes in pediatric HL, or if a different gene expression set is needed.25
In the future, a more complete understanding of the molecular pathogenesis of pediatric HL will provide clues to new and better treatments directed toward tumor-specific molecular lesions. Flow-sorting tissue for H/RS and intratumor T cells and optimizing low-input exome sequencing is beginning to overcome the limitations of genomic evaluation in HL.26 Among 10 patient samples including 2 obtained from pediatric patients, inactivating mutations in β-2-microglobulin were identified as the most commonly altered gene in H/RS cells, leading to loss of MHC class I expression, which is essential for recognition of antigen by CD8 cytotoxic T cells.
In addition, genome wide association studies (GWAS) have provided information on both genetic susceptibility to develop HL, as well as risk for developing late complications, including second malignant neoplasms. A recent meta-analysis totaling 3097 cases and 11 095 controls in the combined discovery and replication sets noted associations between HL subtypes and loci at 2p16, 5q31, 6p31, 8q24, and 10p14 and 19p13.3.27 These GWAS studies also underscore the role of the MHC in disease etiology by revealing a strong human leukocyte antigen (HLA) association. A genetic locus associated with risk for second cancers in HL survivors has also been identified by GWAS.28 This association with a locus on chromosome 6q21 was observed in another set of patients receiving RT for HL during adolescence but not in patients with HL treated as adults, consistent with the epidemiologic observation that radiation-related cancer risks may be higher for individuals exposed at younger ages. The risk locus was found to regulate induction of a transcriptional repressor, PRDM1, by ionizing radiation. Approximately 30% of survivors of HL with the risk haplotype developed a second cancer within 30 years versus only 3% with the protective haplotype. Moreover, that 50% of Caucasians are homozygous for the risk variant suggests that common variants may have very large effects but only in the context of specific exposures such as radiotherapy.
Novel agents investigated in pediatric trials
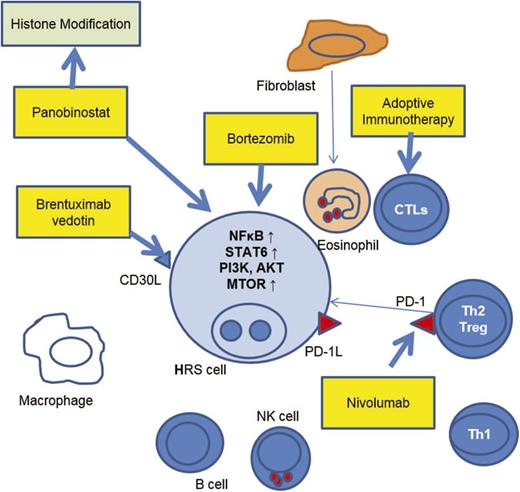
The past several years has seen the emergence of novel therapeutics that truly target the pathology of HL. Agents have focused on the differential expression of cell surface antigens such as CD30, oncogenic dependence of H/RS cells on activated intracellular signaling pathways and induction of immune responses targeting the H/RS cells through modulation of the microenvironment. Most of the information on these agents has been gained through trials in adults with HL,29 however, there have been several studies from which pediatric HL specific data is available, and there are several important trials in progress specifically for children and adolescents with HL. Information on these agents is reviewed here (Figure 1).
Therapeutic targets in the HRS cell and immunoreactive cells in the surrounding microenvironment. Agents highlighted in boxes indicate agents with pediatric Hodgkin lymphoma-specific data, or pediatric trials in progress.
Therapeutic targets in the HRS cell and immunoreactive cells in the surrounding microenvironment. Agents highlighted in boxes indicate agents with pediatric Hodgkin lymphoma-specific data, or pediatric trials in progress.
Most H/RS cells strongly express CD30, a member of the TNF cell receptor superfamily that has highly restricted expression in normal cells. After several largely unsuccessful efforts to develop an anti-CD30 antibody therapy, brentuximab vedotin (Bv), an anti-CD30 antibody linked to a cytotoxic agent, monomethyl aurostatin E (MMAE) has been shown to have significant promise in both the relapsed and upfront therapy settings.30,31 Bv has been shown to kill H/RS cells through the intracellular release of MMAE, disrupting the microtubule network and leading to G2/M cell cycle arrest and apoptosis. The FDA has approved Bv for adult patients with HL after failure of autologous stem cell transplant (ASCT) or after failure of at least 2 prior multi-agent chemotherapy regimens in patients who are not candidates for ASCT. Post-ASCT maintenance therapy with Bv has recently been found to prolong progression-free survival in adults with recurrent HL.32 An international randomized phase 3 trial is comparing ABVD versus AVD with Bv for adults with advanced stage HL.
Pediatric experience with Bv is more limited. Brentuximab vedotin is generally well tolerated in pediatric patients at a dose of up to 1.8 mg/kg every 3 weeks. Five patients aged 12-17 with HL were enrolled on the pivotal phase 2 trial that led to the FDA approval and there were no common severe toxicities or premature discontinuation of therapy related to an adverse event.33 Ten patients aged 2 to <18 years with relapsed or refractory HL received single agent Bv in an international pediatric phase I study.34 A phase 2 study is in progress (NCT 01492088). The combination of Bv with gemcitabine is being investigated in a COG phase 1/2 trial for relapsed/refractory HL up to age 30 years (NCT01780662). Results from the phase 1 part also confirm that the combination is generally well tolerated, and the phase 2 part is recruiting participants. Given the status of these trials, it is important to emphasize that Bv is not standard of care for first relapse.
Combination therapy with Bv in the frontline setting in high-risk pediatric HL is being investigated in 2 active trials sponsored by major consortia. In a pilot phase 2 study, the Pediatric HL Consortium is investigating the early response rate and progression free survival of Bv as a substitute for vincristine in the European OEPA/COPDac regimen (NCT01920932). In a 600 patient randomized phase 3 trial, COG is evaluating the efficacy of Bv as a replacement for bleomycin in the ABVE-PC regimen (NCT02166463).
Among the more promising approaches to activating therapeutic antitumor immunity in HL is the blockade of the immune checkpoint, programmed cell death protein 1 (PD-1) pathway. Classical HL is characterized by H/RS cells surrounded by an extensive but ineffective inflammatory cell infiltrate. Increased PD1 expression by T lymphocytes in the microenvironment and increased PD1 ligand expression by H/RS cells allow evasion of T cell–mediated destruction of H/RS cells. Blocking the interaction between PD-1 and its ligands through the administration of PD-1 blocking antibodies can result in T-cell activation and a more florid tissue inflammatory response.35 Nivolumab is associated with a high objective response rate of 87% among heavily pretreated patients with HL.36 The COG is investigating single agent nivolumab in relapsed/refractory HL (NCT 02304458). Another anti–PD-1 drug, pembrolizumab, was associated with a 66% response rate in a similar cohort of adult patients with HL.37 Current and planned studies in adults with HL are now investigating these agents in conjunction with other targeted therapies, including brentuximab vedotin and ipilimumab, a monoclonal antibody directed against cytotoxic T lymphocyte-associated antigen-4 (CTLA4), an antigen that is expressed on activated T cells and exhibits affinity for B7 costimulatory molecules (NCT01896999).
Targeted T-cell therapies are being explored for pediatric HL patients associated with EBV either as adjuvant therapy after transplant or for relapsed disease. Autologous cytotoxic T lymphocytes (CTLs) directed against the type II latency Epstein–Barr virus (EBV) antigens, latent membrane protein 1 (LMP1) and LMP2, have been associated with clinical responses in patients with active disease, as well as potentially helping to maintain continued remission when used in the adjuvant setting in patients at high risk for relapse.38 Of the 50 patients enrolled on a recently reported study, 11 patients with HL were < 21 years of age. This approach is limited by difficulties in obtaining adequate autologous derived CTLs from patients who generally have been treated with multiple cycles of chemotherapy as well as the 1-2 month time frame required to prepare the CTLs. A recently activated trial is evaluating the safety of allogeneic CTLs obtained from banked blood of healthy donors and made specifically to react to 3 EBV proteins, LMP1, BamHI-A rightward frame-1 (BARF1) and EBV nuclear antigen 1 (EBNA1; NCT 02287311). Eligibility includes pediatric patients with relapsed HL. Laboratory studies in cultured lymphoma cell lines also suggest that other immune targets, such as cancer testes antigen (CTA), may be amenable to CTL directed approaches for patients with HL not associated with EBV.
Nuclear factor (NF)-kB activation is a molecular hallmark of the H/RS cell and given its role in growth, survival, and immunomodulatory functions of the cell is an attractive target for therapy. Bortezomib is a small molecule that inhibits the 26S proteasome, stabilizing proteins degraded by the ubiquitin-proteasome system including the NF-κB inhibitor, IκB. A COG trial aimed to determine if bortezomib (B) increased the efficacy of ifosfamide and vinorelbine (IV) in pediatric HL.39 This study enrolled 26 relapsed HL patients (<30 years) treated with 2 to 4 cycles of IVB. The primary endpoint was anatomic CR after2 cycles. Secondary endpoints included overall response (OR: CR + partial response) at study completion compared with historical controls [72%; 95% confidence interval (CI): 59%-83%]. Although few patients achieved the primary objective, OR with IVB improved to 83% (95% CI: 61%-95%; p = 0·32). However, considering these results in conjunction with results from recent adult trials in which the addition of bortezomib to ifosfamide, etoposide, and carboplatin40 or to dexamethasone41 did not improve response rates leads to reduced enthusiasm for further study of bortezomib in HL.
Epigenetic therapies, such as the histone deacetylase (HDAC) inhibitors have demonstrated potential therapeutic benefits in adults with HL; these agents work via multiple mechanisms, leading to both anti-tumor and immunoregulatory effects.29 No published data is available in pediatric HL, although panobinostat is being evaluated in a phase 1 trial for children with relapsed/refractory hematological malignancies including HL (NCT01321346).
Implications for future study design
In summary, most children with HL are initially treated with risk-adapted chemotherapy alone or in combination with low dose involved field or node RT to achieve local disease control while minimizing bystander organ toxicity, although there is no single standard of care approach. For patients that relapse, several different chemotherapy regimens are used to maximize complete response prior to consolidation therapy with AHSCT. Although several of the novel targeted agents are showing promising activity, many questions remain in determining how best to use these agents: Can they replace conventional cytotoxic agents and RT that have broader organ toxicity, especially in the pediatric population? How can these novel agents be used in combination? Are there predictive biomarkers that can be used to define earlier endpoints of progression free survival or risk for late organ toxicities?
As has been highlighted in many other areas of cancer drug development, the identification of active targeted therapy combinations or targeted therapies in conjunction with conventional agents requires novel hypothesis-testing, biomarker-driven clinical trial designs to optimize both drug dosing and scheduling, and should ideally be supported by emerging computational and experimental network biology science. Thus, although there is rationale for testing these agents in HL, there are many significant barriers that will hinder their study and eventual application to clinical practice. Given the low prevalence of the disease, overall favorable prognosis with conventional therapies, unavailability of strong preclinical testing models, and inadequately validated biomarkers, progress is likely to be slow. Collaborations with both international pediatric HL research groups, as well as with the adult cooperative groups, especially for the AYA population, will be of significant benefit in moving the field forward.
In the interim, collaborations aimed at improving risk stratification for optimal allocation of chemotherapy and especially radiotherapy will help to improve the therapeutic index. Harmonization of staging and response criteria will also allow comparison of trial results and likewise facilitate more rapid integration of new initiatives into clinical practice.
This article was selected by the Blood and Hematology 2015 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2015. It is reprinted in Hematology Am Soc Hematol Educ Program. 2015;2015:514-521.
Authorship
Contribution: K.M.K. wrote the paper.
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence: Kara M. Kelly, Columbia University Medical Center, Division of Pediatric Hematology/Oncology/Stem Cell Transplantation, 161 Fort Washington Ave, IP-7, New York, NY 10032; e-mail: kk291@columbia.edu.