Abstract
Introduction. Heparin is widely used as an anticoagulant to prevent thrombosis and to treat venous thromboembolism and myocardial infarction. A complication of heparin use is the development of heparin-induced thrombocytopenia (HIT), which is a limb- and life-threatening disorder due to associated thrombotic events. HIT arises through the formation of immune complexes between heparin, platelet factor 4 and HIT autoantibodies. These immune complexes engage with FcγRIIa receptors on platelets, leading to platelet activation and aggregation and subsequent initiation of the coagulation pathway. Current HIT treatment consists of cessation of heparin administration and substitution with parenteral anticoagulants such as argatroban and danaparoid. While these anticoagulants are generally beneficial in reducing thrombocytopenia, they are only partially effective since the risk of thrombosis continues due to the underlying FcγRIIa-mediated platelet activation. Thus, alternative anticoagulants do not reduce morbidity and mortality rates, highlighting the need for more effective HIT interventions.
Methods. IV.3 is a monoclonal antibody that recognizes and blocks the FcγRIIa receptor and is used in assays to confirm the presence of HIT antibodies. We derived the VH and VL sequences of IV.3 and constructed a single-chain variable fragment (scFv) antibody in the form of VH-linker-VL. Using a complementarity determining region grafting and point mutation approach the scFv was humanized with the aim of reducing potential immunogenicity for future clinical applications. The molecule was expressed in E. coli and purified by FPLC. We reconstituted the HIT condition in a micro-fluidics device on a Vena8 Fluoro+ biochip coated with vWf using whole blood flowing at 20 dyne/cm2 at 37oC. Whole blood was stained with DiOC6 and the formation of platelet aggregates was monitored by fluorescence microscopy. Video images were acquired at 1 frame every 2 sec for 460 sec.
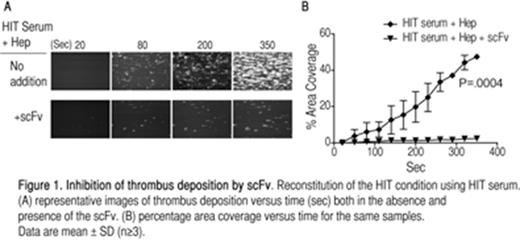
Results. The purified scFv interacts with FcγRIIa on platelets. Platelet aggregation and serotonin release assays show that the scFv effectively prevents aggregation and activation induced by HIT immune complexes. We demonstrate that in the HIT condition reconstituted in a micro-fluidics system the scFv precludes thrombus deposition in a dose-dependent manner as determined by thrombus coverage area and mean thrombus diameter (Figure 1).
Conclusions. These data provide evidence that a humanized scFv binds and neutralizes FcγRIIa on platelets. This interaction prevents HIT immune complex-induced platelet aggregation and activation in vitro and stops thrombus deposition ex vivo. This molecule, therefore, inhibits a critical initiating event in HIT and may serve as a potential treatment for this condition.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal