Abstract
Background: Immunochemotherapy has improved the clinical outcome of patients diagnosed with diffuse large B-cell lymphoma (DLBCL), but only 60% of all DLBCL patients are potentially cured. A response-adaptive imaging strategy that accurately determines the initial response to therapy could improve clinical outcomes. Although there has been an increasing trend to perform interim PET/CT to monitor response, the optimal interpretation method for interim PET analyses remains uncertain. Studies using maximum standard uptake value (SUVmax), have not defined a uniformly applicable SUVmax reduction cutoff that accurately predicts clinical outcome. Here, we hypothesized that a method that maximized the detection of all metabolically active regions within the tumor mass, defined as the metabolic tumor volume (MTV), could serve as a better predictor of clinical outcome than SUVmax measurement.
Methods: All patients with DLBCL that were treated from Dec 2006 to Dec 2014 at the University of Cincinnati were studied, retrospectively. Interim PET analysis was performed after 2-4 cycles of chemotherapy. SUVmax and MTV on the initial and interim PET CT for each patient was determined. To evaluate the contribution of metabolic activity within the tumor periphery in assessing clinical outcomes, MTV was measured by two separate methods: fixed-threshold and gradient-segmentation using MIMSoftware, OH, USA. The primary end point of the study was progression free survival (PFS). To identify an optimal threshold cutoff that could predict PFS more accurately, the receiver operating characteristic (ROC) curve analysis was used.
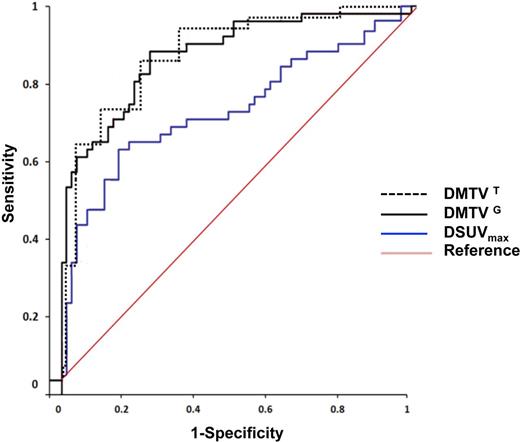
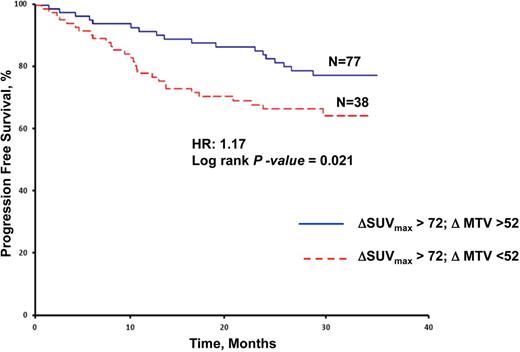
Results: A total of 197 patients with pathology confirmed diagnosis of DLBCL were recognized. Of the 197 patients, 167 underwent interim PET analysis. All patients fasted >6 h before intravenous injection of 18F-glucose, had glucose levels >90 and <160 mg/dl at the moment of injection, scans were performed within 90 min after injection and granulocyte-colony stimulating factor was stopped >48 h before imaging. The median follow-up period for patients in the study was 37 months. R-CHOP (Rituximab, Cyclophosphamide, doxorubicin/Hydroxydaunomycin, vincristine/Oncovin and Prednisone) and R-DA-EPOCH (Dose-Adjusted Etoposide, Prednisone, Oncovin, Cyclophosphamide and Hydroxydaunorubicin) were the first line of therapy in 74% and 26% of patients, respectively. On interim PET/CT, 69% of patients achieved complete response with the remaining patients showing partial response based on visual assessment. Dichotomous visual interpretation of interim PET did not correlate with PFS (log-rank P= 0.37). Compared with the threshold-based method, the gradient-based method resulted in a statistically significant greater MTV in pretreatment, as well as interim PET images. However, no significant difference was noted between the reduction in MTV determined by the threshold-based (ΔMTVT) or gradient-based (ΔMTVG) methods (median 34% vs 36%, P =0.29). Thresholds of ΔSUVmax and ΔMTV by ROC curve were 72% and 52%, respectively. ΔMTV predicted PFS better than ΔSUVmax as the AUC for ΔMTV was significantly larger compared with that for ΔSUVmax (Figure 1). All patients who achieved a SUVmax reduction greater than the cutoff value determined by the ROC analysis (ΔSUVmax>72%) were then stratified into two groups based on a ΔMTV cutoff value > or <52%. From a total of 115 patients who achieved a ΔSUVmax >72% on interim PET/CT imaging, 77 (67%) had a ΔMTV >52%. Importantly, patients who achieved a ΔMTV >52% had a statistically significantly greater PFS compared with patients who achieved a ΔMTV <52% (Figure 2). Among 115 patients who achieved a ΔSUVmax >72% on interim PET and those who demonstrated a ΔMTV >52% exhibited greater PFS.
Conclusion: Our study highlights the importance of MTV assessment for patients who achieved background SUVmax on the interim PET to better predict the clinical outcome of DLBCL patients. The MTV measurement with the gradient-based method renders a larger volume of tumor than the threshold-based method; however, these two methods report a similar percentage reduction and are interchangeable in terms of interim PET interpretation. Metabolic activity of the peripheral area of the tumor should be incorporated into response-adaptive strategies and prospective trials that evaluate the response to current and novel therapeutic regimens to treat DLBCL patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal