Abstract
Introduction
The Myeloproliferative Neoplasms (MPNs) are characterized by a recurrent point mutation of JAK2 gene (JAK2 V617F). This mutation, which usually affects only one of the JAK2 gene alleles in Essential Thrombocythemia (ET), frequently becomes homozygous in Polycythemia Vera (PV) and Myelofibrosis (MF) due to homologous mitotic recombination. A JAK2 V617F-mutated disease is strongly associated with a specific constitutional JAK2 haplotype, designated 46/1(GGCC), (Nat Genet 2009; 41:446) with complete linkage disequilibrium although the risk of developing MPNs is independent of the 46/1 haplotype. Furthermore, JAK2 V617F specifically arises on the 46/1 allele in most cases, thus predisposes to the development of MPN (Leukemia 2010; 24:1533; Nat Genet 2009; 41:446). These observations suggest that it is possible to define the JAK 2V617F clonal architecture starting from 46/1 SNP allele burden and the homologous recombination frequency.
Aim and Methods The aim of the study was to investigate changes in the JAK2 V617F clonal structure in patients affected by PV, ET or MF and treated with ruxolitinib, a JAK1/JAK2 inhibitor recently approved for MF and PV and under investigation in ET pts intolerant of or resistant to hydroxyurea. We used a recently described methodology (Leukemia 2014;28:460) combining allele burden evaluations of both 46/1 and JAK2 and the frequency of mitotic recombination to derive the percentages of JAK2 V617F clones in MPN patients in 46/1 heterozygous patients for rs12343867 polymorphism (C/T). The JAK2 allele burden values were confirmed independently by two RTQ-PCR methods, according to Lippert (sensitivity, 0.8%) and to Larsen (sensitivity, 0.08%) method.
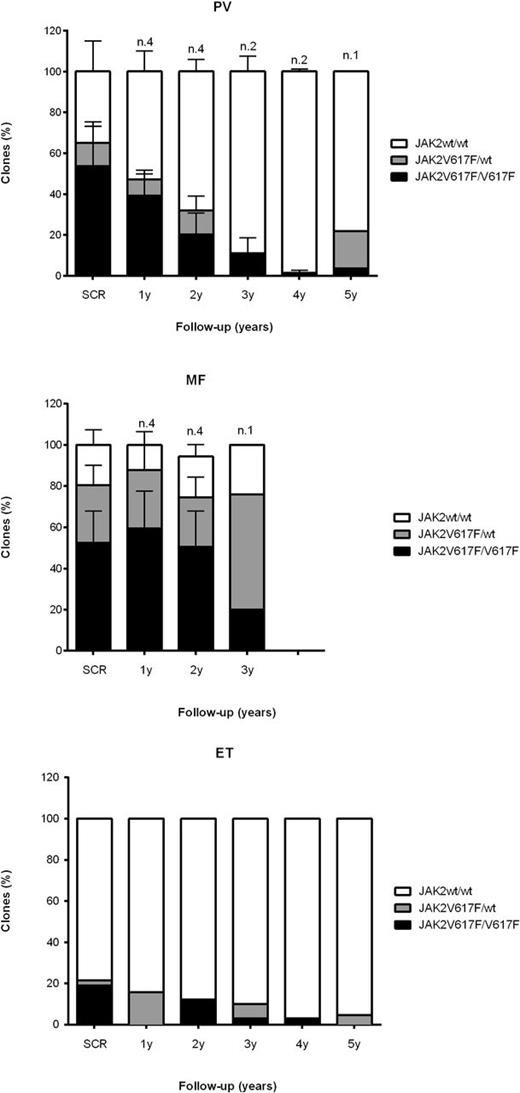
Results We analysed 40 patients (pts) affected by MPNs (12 PV, 1 ET and 27 MF) treated with ruxolitinib over 1 to 5 years (yrs) of follow up (FU); 13 patients (32.5%) presented a reduction of at least 10% of JAK2 V617F allele burden values at latest FU: 7/12 PV (58.3%), 1/1 ET (100%), 6/27 PMF (22.2%). Among these, 9 pts with at least two years of FU were eligible for the study. A quantification of JAK2 homozygous (V617F/V617F), heterozygous (JAK2 V617F/wt) and wild type (wt/wt) clones was obtained using the methodology described above. We found a median reduction of JAK2 V617F/V617F and JAK2 V617F/wt clones of 32.31 % and 8.82 %, respectively, in PV (n.4); 12.22% and 17.86%, respectively, in MF (n.4); 35.99% and 100%, respectively, in ET (n.1). Furthermore, an almost complete molecular remission (CMR) was seen in two PV patients with 4 and 5 yrs of FU respectively and in one ET patient after 5 years of treatment. In these patients we observed reduction of homozygous clones of 99.60%, 86.91% and 100%, respectively, and the residual JAK2 allele burden was due to the persistence of JAK2 V617F/wt clones (Figure). Conversely, in one patients with an increase in JAK2 allele burden from 39% to 45% after 1,5 yrs under ruxolitinib, we observed an increase in JAK2V617F/wt but not in JAK2 V617F/V617F clones.
Conclusion. Taken together, these results showed that ruxolitinib may preferentially target the homozygous clones inducing in some cases an almost complete molecular remission with prolonged treatment. Future analysis will be performed to study the clonal architecture in an increased number of patients treated with JAK inhibitors as single agent or in combination with other drugs.
Vannucchi:Shire: Speakers Bureau; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal