Abstract
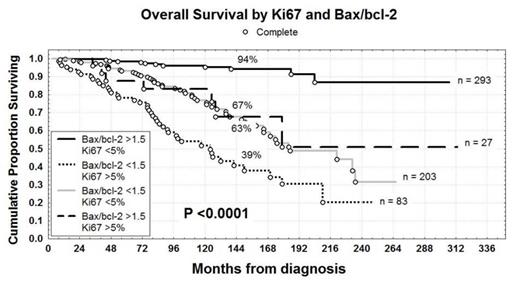
Differences in tumor proliferation and apoptosis levels explain the heterogeneous clinical course of CLL reflecting both genetic differences and the activity of external signals mainly through the B-cell receptor pathway (Herishanu et al, Blood, 2011; Rossi et al, Blood, 2013). Therefore high dynamic proliferation rate and impaired apoptosis represent crucial mechanisms both in the chemoresistance and in the progressive disease (Messmer et al, J Clin Invest 2005; Cervantes-Gomez et al, Clin Cancer Res, 2015). The today availability in clinical use both of ibrutinib, a potent Bruton tyrosine kinase (BTK) inhibitor, which blocks cell proliferation and trafficking (Cheng S et al, Leukemia, 2013; Burger et al, Blood, 2014) and venetoclax (ABT-199), a novel potent oral anti-bcl-2 peptidomimetic (Seymour et al, J Clin Oncol, 2014) as well as their possible synergistic combination (Portell et al, Blood, 2014), prompted us to analyze the real impact of the proliferative rate (Ki67 percentages) and the apoptosis (bax/bcl-2) on CLL prognosis. The primary aims of our research were: 1) to correlate Ki67 and bax/bcl-2 with clinical and biological prognostic factors; 2) to determine progression free survival (PFS) and overall survival (OS) upon Ki67 and bax/bcl-2; 3) to evaluate Ki67 and bax/bcl-2 as independent prognosticators. Therefore we investigated 606 patients, median age 66 years (range 33-89), 340 males and 266 females. With regard to modified Rai stages at diagnosis, 220 patients had a low stage, 369 an intermediate stage and 17 a high stage. Ki67 was evaluated by flow cytometry in terms of percentages of CD19+CD5+ CLL cells and the threshold of positivity was set at >5% (range 0.10-22.7). Also bax/bcl-2 was calculated by flow cytometry, dividing mean fluorescence intensity (MFI) of bax by MFI of bcl-2 on CLL cells. The threshold was set at the median value >1.5 (range 0.27-6.10). One hundred-ten patients were Ki67+ (18.2%) and 321 were bax/bcl-2+ (53%). Both higher Ki67 and lower bax/bcl-2 were significantly associated with intermediate/high Rai stage, beta-2 microglobulin (B2M)>2.2 mg/dl and soluble CD23>70 U/ml (P<0.0001). Moreover, higher Ki67 and lower bax/bcl-2 were greatly represented within the high risk (del11q or del17p) cytogenetics (P<0.0001). Noteworthy, strong correlations were found between higher Ki67 or lower bax/bcl-2 and IGHV unmutated status (P<0.0001) or NOTCH1 (P<0.0001 and P=0.00003) or TP53 mutations (P<0.0001 and P=0.00001). With regard to clinical outcome, significant shorter PFS and OS were observed in patients with higher Ki67 (2% vs 44% and 43% vs 83% at 12 years; P<0.0001) and lower bax/bcl-2 (13% vs 57% and 58% vs 91% at 12 years; P<0.0001). Noteworthy, bax/bcl-2 and Ki67 showed synergistic prognostic properties, since bax/bcl-2>1.5 plus Ki67<5% identified a CLL subset at best prognosis with regard to OS (94% vs 39% at 12 years; P<0.00001, Figure), so suggesting that apoptosis and proliferation may be key pathways for combined targeted therapies. The two discordant subsets (bax/bcl-2<1.5/Ki67<5% and bax/bcl-2>1.5/Ki67>5%) showed an intermediate outcome (67% and 63% at 12 years, respectively, Figure). In multivariate analysis of OS (593 patients), bax/bcl-2 ratio (P=0.004) and Ki67 (P=0.02) together with age (P=0.0002), B2M (P=0.005), cytogenetics (P=0.003), IGHV status (P=0.0005) and TP53 (P=0.0006) were confirmed as independent prognostic factors. Therefore, both the proliferative marker Ki67 and the apoptotic index bax/bcl-2, performed by flow cytometry, are powerful prognosticators showing synergistic clinical effects. Experiments of apoptosis, proliferation and trafficking testing ABT-199 and ibrutinib on CLL cells in vitro are in progress at our Institutions (Gattei et al, unpublished data). In conclusion, the modern treatment strategies in CLL should aim to block proliferation and trigger apoptosis concurrently (i.e. ibrutinib and venetoclax) in order to achieve longer overall survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal