Abstract
Background: Bortezomib has become an integral part of front-line therapy of multiple myeloma in a large majority of patients. There are preliminary reports which show that addition of bortezomib can augment the peripheral blood CD34 count during stem cell mobilization. In this single center prospective trial we added bortezomib to G-CSF to evaluate the effects of bortezomib on peripheral CD34 counts and collection.
Methods: Patients aged 18-70 years with diagnosis of multiple myeloma (MM) or non-hodgkin's lymphoma (NHL) who were eligible for autologous stem cell transplantation (ASCT) and had received no more than three prior chemotherapeutic regimens were eligible for the study. Patients were enrolled in two groups. Group A (N=3) received G-CSF 16mcg/kg for 5 days and proceeded to stem cell collection on D5 and then received bortezomib 1.3mg/m2 on D5 after stem cell collection and G-CSF 16mcg/kg on D6, 7, 8 and repeat stem cell collection on D6, 7, 8 till the goal was achieved. Group B (N=17) received G-CSF 16mg/kg on D1-5 and received bortezomib 1.3mg/m2 on D4 and proceeded to stem cell collection on D5. If the patient was not able to collect the predefined goal CD34, G-CSF was continued on D 6, 7, 8 and a second dose of bortezomib 1.3mg/m2 was given on D7. Mobilization procedure was stopped once the predefined goal CD34 collection (4 x 106/kg for MM and 2 x 106/kg for NHL) had been collected.
Primary objectives of the study was to determine if addition of bortezomib to G-CSF will result in an increase in PBSCs by > 2-fold and to achieve median neutrophil engraftment 12 days post ASCT. Secondary objectiveswere to evaluate the collected product for co-mobilization of lymphoma or myeloma cells and to determine if the use of bortezomib increases the mobilization of immune-stimulatory Dendritic cell (DC) -1 subsets.
Results: A total of 23 patients were enrolled and 20 were evaluable for the results. Only one patient with NHL was enrolled and rest had MM. Median age of pts was 57 years, M/F 8/12, median number of previous chemotherapy regimens was 1 (range 1-3).
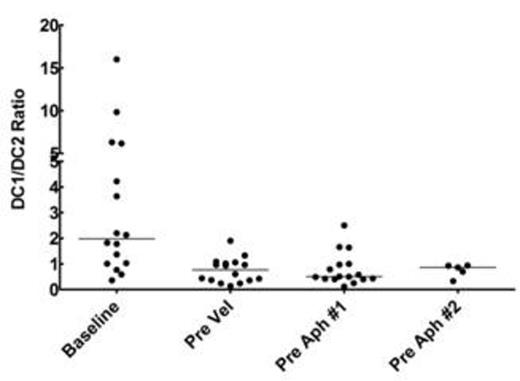
The median peripheral blood CD34 count pre and post bortezomib in all patients were 28.8 x 106/kg and 37 x 106/kg respectively. All three patients in group A had drop in peripheral blood CD34 counts on D6 post bortezomib as they had undergone stem cell collection on day 5. In part B (N=17), 15 patients had increase in peripheral blood CD 34+ve cell counts with 4 patients achieved doubling while 11 pts had less than doubling of peripheral blood CD34 count after receiving bortezomib. Two patients had minimal drop in the peripheral blood CD34 counts post bortezomib. Median number of CD34 cells collected in15 patients (part B) were 5.06 x 106 CD34 cells/kg (range 4-15.1). 18 patients proceeded to ASCT and median time to neutrophil engraftment (ANC ≥500/cumm) post transplant was 12 days (range 11-16) and platelet engraftment (Plt count ≥ 20,000/cumm) was 18 days (range 15-27). There was no significant change in DC1/DC2 ratio in both groups following treatment with bortezomib and G-CSF (Figure 1).
In group A all three patients collected goal CD34 count on day 5 and 2/3 patients collected >4 x106 CD34 cells/kg on D6 post bortezomib and1/3 patients collected 2.6 x 106 on D6 post bortezomib. In group B (n=17), 2 patients were unable to collect because of low CD34 counts on D4 and D5, 11 pts collected the goal in one day (D 5) and 4 pts required two days of apheresis (D 5 and 6). None of the patients received D7 bortezomib.
Conclusion: Use of bortezomib during autologous stem cell collection was safe and well tolerated. Majority of patients had increase in peripheral blood CD34 counts post bortezomib administration on D4. Future trials should explore bortezomib as an alternate strategy to chemo-mobilization in combination with growth factors.
Off Label Use: Bortezomib for stem cell mobilization. Lum:Karyopharm Therapeutics Inc: Equity Ownership; Transtarget.Inc: Equity Ownership. Deol:Bristol meyer squibb: Research Funding. Abidi:celgene: Speakers Bureau; Millenium: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal