Abstract
Introduction. This was a cross-sectional study of patients with hemoglobinopathies attending 13 Italian centers participating in the LICNET (Liver Iron Cutino Network) network promoted from Piera Cutino partnership and instituted by our center (Campus of Haematology Franco e Piera Cutino-A.O.O.R. Villa Sofia Cervello, Italy) on February 2013. LICNET is addressed to the diagnostics of iron overload in liver by R2 MRI (Ferriscan®) in patients with hemoglobinopathies. Ferriscan is a rapid scan method now available (10 minutes). This tool is crucial to have accurate and reliable measures for iron body burden control in hemoglobinopathies.
Methods. Data included patients with β-thalassemia major (TM) (regularly transfused) (TX), β-thalassemia intermedia (TI) (both TX and non-transfused) (non-TX), and sickle cell disease (SCD) (both TX and non-TX). The main aim of the study was to evaluate how serum ferritin levels (SFLs) predict liver iron concentration (LIC) in different hemoglobinopathies, and to have valuable information about prognosis and response to therapy. In particular, to identify SFLs that best predict LIC thresholds of clinical significance (7 and 15 mg Fe/g dw) by identifying levels with highest sum of sensitivity and specificity was used the receiver operating characteristic (ROC) curve analysis.
Results. A total of 363 patients were evaluated in this analysis, with a mean age of 35.6 ± 13.0 years (range: 6-76) and including 160 (44.1%) males. The underlying diagnosis were β-TM (n=204, 56.2), β-TI (n=102, 28.1%), and SCD (n=57, 15.7%). Among β-TI patients, 60 (58.8%) were on transfusion therapy. Similarly, in patients with SCD 34 (59.6%) were on transfusion therapy. The mean LIC in the study population was 7.8 ± 9.6 mg Fe/g dw and the median was 4.0 mg Fe/g dw.
Across underlying diseases, LIC distribution was as follows: β-TM (mean: 9.0 ± 10.7, median: 4.9 mg Fe/g dw), TX β-TI (mean: 7.1 ± 7.3, median: 5.0 mg Fe/g dw), non-TX β-TI (mean: 5.1 ± 6.0, median: 3.2 mg Fe/g dw), TX SCD (mean: 8.5 ± 11.0, median: 4.5 mg Fe/g dw), and non-TX SCD (mean: 3.1 ± 1.9, median: 2.4 mg Fe/ g dw). It was apparent that TX patients irrespective of underlying diagnosis have a comparable proportion of patients with high LIC risk categories (>7 mg Fe/g dw) (p=0.627).
Among chelated patients, LIC distribution was as follows: Deferoxamine (DFO) (mean: 7.3 ± 8.5, median: 4.7 mg Fe/g dw), Deferiprone (DFP) (mean: 11.6 ± 11.4, median: 8.4 mg Fe/g dw), Deferasirox (DFX) (mean: 7.8 ± 10.3, median: 3.2 mg Fe/g dw), DFO+DFP (mean: 8.2 ± 10.6, median: 4.5 mg/ Fe g dw), and other combinations (mean: 6.5 ± 4.0, median: 5.1 mg Fe/ g dw), with a statistically significant difference noted between groups (p =0.009) with the highest median among chelated patients noted in DFP treated patients and lowest median noted in DFX treated patients.
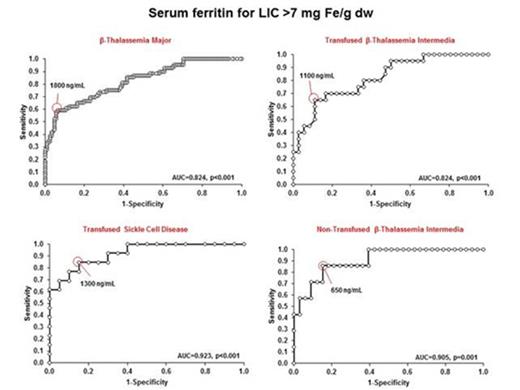
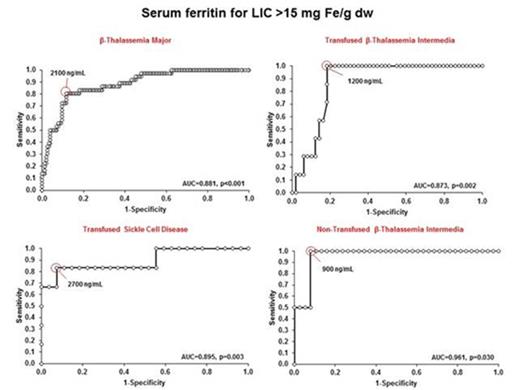
For underlying disease groups, ROC curve analysis showed that SFLs that best predict LIC thresholds of 7 and 15 mg Fe/g dw varied, although patients with β-TI showed lowest SFLs to predict these thresholds especially non-TX patients (Fig. 1, Fig.2).
Discussion. This study suggest as high values of LIC are present even in patients with TI or SCD, confirming that gastro-intestinal iron absorption is one of the main mechanism for secondary iron overloading. Moreover, close to 20% of patients with non-TX β-TI continue to have high LIC thresholds, while none of non-TX patients with SCD have LIC values > 7 mg Fe/g dw. The evidence that SFLs of 900 ng/mL are related in β-TI with LIC > 15 mg Fe/g dw (Fig. 2) suggests as chelation treatment could be reconsidered earlier in this cohort of patients. Finally, these findings suggest as LIC is predicted by different SFLs according to the different types of hemoglobinopathy.
Receiver operating characteristic curve analysis of serum ferritin level for predicting LIC>7 mg Fe/g dw in Thalassemia Major, Thalassemia Intermedia and Sickle Cell Disease patients.
Receiver operating characteristic curve analysis of serum ferritin level for predicting LIC>7 mg Fe/g dw in Thalassemia Major, Thalassemia Intermedia and Sickle Cell Disease patients.
Receiver operating characteristic curve analysis of serum ferritin levels for predicting LIC>15 mg Fe/g dw in Thalassemia Major, Thalassemia Intermedia and Sickle Cell Disease patients.
Receiver operating characteristic curve analysis of serum ferritin levels for predicting LIC>15 mg Fe/g dw in Thalassemia Major, Thalassemia Intermedia and Sickle Cell Disease patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal