Abstract
Introduction: Adult T-cell leukemia-lymphoma (ATL) is a peripheral T-cell malignancy, which is caused by human T-cell leukemia virus type I (HTLV-1) and is considered to be derived from regulatory T cells (Treg). ATL is usually resistant to conventional chemotherapies and the patients with ATL have very poor prognosis. Mogamulizumab (Moga) is a humanized monoclonal antibody against CC chemokine receptor 4 (CCR4) which is expressed on T-helper type 2, Treg and tumor cells from most patients with ATL. It was reported that Moga used to treat ATL with 50% efficacy in a phase II study and frequently developed cutaneous adverse reactions (CAR). In that study, development of CAR was reported to be closely associated with the response to Moga therapy. To confirm efficacy and safety of Moga therapy for ATL, we retrospectively analyzed relapsed or refractory ATL patients who were treated by Moga at 4 institutes in Kagoshima, one of the endemic areas of HTLV-1 infection, Southwestern Japan.
Patients and methods: There were 77 patients, who were received Moga therapy between March, 2007 (phase I study) and December, 2014. We studied about backgrounds (age, sex, subtypes of ATL, ECOG performance status (PS) at diagnosis and clinical stage) of the patients, contents of prior therapy and the response, prognostic index for acute and lymphoma type ATL (ATL-PI), PS and disease status at Moga therapy, response and adverse events (AE) of Moga therapy, and the survival.
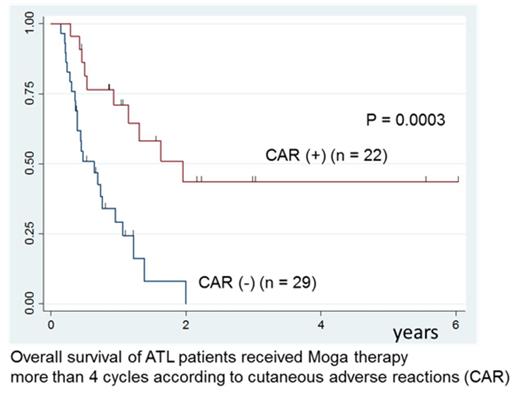
Results: Enrolled patients were 44 men and 33 women, and median age at Moga therapy was 65.6 years (range: 44-83 years). They consisted 54 acute, 18 lymphoma, and 5 chronic type ATL, and ATL-PI was low in 18, intermediate in 43 and high in 16 patients. Initial induction chemotherapies were mainly VCAP-AMP-VECP regimen in 41, CHOP like regimen in 19 patients. Median duration between diagnosis and Moga therapy was 8.1 months (range: 0.5-94.9 months). They were administered Moga median 5 cycles (range: 1-10 cycles). Disease status at Moga therapy were 23 relapsed and 54 refractory [partial response (PR): 8, stable disease (SD): 15, progressive disease (PD): 31] disease. Myelosuppression associated with Moga, development of cytomegalovirus antigenemia (19.5%) and other AE were tolerable. Best objective response rate was 42.9% including 18 complete remission (CR) and 15 PR. Fifty-four patients died after Moga therapy (50 from ATL, 4 from infection and so on). Median overall survival (OS) time from Moga therapy was 7.7 months (95%CI: 5.1-11.2 months) and 3-year OS rate was 18.2%. The cohort included 27 patients over 70 years, but their survival was no worse than younger. Twelve patients received allogeneic hematopoietic stem cell transplantation after Moga therapy, but had no survival advantage. Twenty-four patients suffered from CAR, which were developed median 7 cycles (range: 2-8 cycles). The severity of CAR according to CTCAE v3.0 was grade 1 in three, 2 in eight, 3 in eleven and 4 in two patients, respectively. In patients (n = 51) received Moga more than 4 cycles, 22 patients suffered from CAR and their OS (3-year OS: 43.6%) was significantly better than patients without CAR (median OS: 23.4 vs 7.9 months, p = 0.0003).
Discussion: Moga therapy is effective and safe for ATL patients and the onset of moderate CAR is a favorable sign of the efficacy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal