Abstract
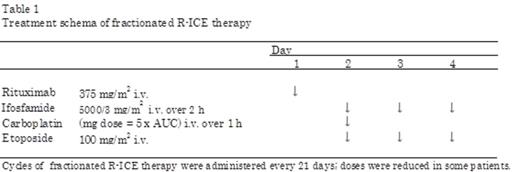
Background: Although many salvage regimens have been used in patients with relapse or refractory lymphoma, these have limitations in elderly or patients with complications due to toxicity and restricted tolerability. In particular, the treatment strategy for patients with severe comorbidity remains to be determined. The combination of ifosfamide, carboplatin, and etoposide (ICE) has been used for relapse and refractory non-Hodgkin lymphoma since the 1990s. Ifosfamide, an alkylating agent, is a key drug in the ICE regimen, which is non-cross resistant with cyclophosphamide. In practice, there are various regimens of ICE treatment. The outpatient-based fractionated regimen of ICE (fractionated ICE) described in 2003 by Herzberg et al involves a different method of ifosfamide-administration from that originally described. Although ifosfamide 5000 mg/m2 is continuously infused for 24 h according to the original ICE regimen, it is infused in three equally divided doses over 2 h on days 1-3 of each cycle in the fractionated ICE regimen. Unlike the original ICE regimen, the modified regimen can be performed in outpatient clinics. The efficacy and tolerability of fractionated ICE as both a salvage and stem cell mobilization regimen have been confirmed in relapse/refractory patients. Many studies have reported the efficacy of the addition of rituximab to chemotherapy for diffuse large B-cell lymphoma patients (DLBCL). However, no study has reported the efficacy of rituximab addition to the fractionated ICE regimen. In this study, we analyzed the efficacy and toxicity of fractionated ICE with rituximab (fractionated R-ICE) as a salvage regimen in relapse/refractory DLBCL patients (Table 1). Further, we compared the response and survival rate after fractionated R-ICE between patients with severe comorbidity and other patients.
Method: We retrospectively analyzed the records of 66 patients with relapse or refractory DLBCL diagnosed between 2000 and 2014. Comorbidities were evaluated using Charlson Comorbidity Index (CCI), and National Cancer Institute Common Toxicity Criteria were used to define toxicities.
Result: Among the 66 patients, 55 received salvage therapies, 30 received fractionated R-ICE, and 25 received it as a second-line salvage therapy. Efficacy and toxicity is demonstrated in Table 2. The overall response rate (ORR) to fractionated R-ICE was 46.7% (n=14) (complete response [CR], 26.7% [n=8], partial response, 20% [n=6]); 1 year survival rate after relapse was 56.7%, and the duration of 50% survival after relapse was 2.4 years. During the cycles, myelosuppression was the most serious toxicity, followed by grade 4 hematological adverse events were 18 (60%) patients, respectively. A previous study showed poor prognosis in patients with high CCIs before the first R-CHOP treatment. In our study, the ratio of patients with chemotherapy dose reduction less than 30% was comparable between low (0 or 1) and high (≥2) CCIs estimated before the fractionated R-ICE therapy. There was no significant difference of survival duration after relapse between low and high CCI (Figure 1). The items used for estimating CCI such as diabetes (n=5), heart disease (n=4), tumor (n=4), and collagen disease (n=3) did not affect survival.
Conclusion: Although patients in our study were old (median: 71 y, range 50-85 y), the CR rate was similar to that of previous studies involving ICE therapy (CR rate 12.5-37%) or R-ICE therapy (CR rate 25-53%). Our results suggest that comorbidities do not have significant impact on the outcome of patients with relapse or refractory DLBCL treated with fractionated R-ICE. Although myelosuppression was severe in patients with high CCI scores, there was no increased incidence of infection or other adverse events. Fractionated R-ICE is supposed to be useful as a salvage therapy, which can be performed in outpatient clinics for relapse/refractory DLBCL patients including those who were older or having high CCI scores.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal