Abstract

Introduction: Anemia represents the main therapeutic challenge in pts with lower-risk MDS (Fenaux P, Adès L. Blood. 2013;121:4280-6). Prospective studies evaluating LEN for the treatment of red blood cell transfusion-dependent pts showed significant clinical activity in both non-del(5q) and del(5q) International Prognostic Scoring System-defined lower-risk MDS (Raza A, et al. Blood. 2008;111:86-93; Santini V, et al. Blood. 2014;124:abstract 409; List A, et al. N Engl J Med. 2006;355:1456-65; Fenaux P, et al. Blood. 2011;118:3765-76). Hematologic adverse events (AEs) are common, but manageable, with LEN treatment (Giagounidis A, et al. Ann Hematol. 2008;87:345-52). However, there has been no direct comparison of safety profiles in non-del(5q) and del(5q) pts. This pooled analysis compared the incidence of AEs in LEN-treated lower-risk MDS pts with or without del(5q).
Methods: This retrospective analysis of pooled data from 7 prospective clinical trials compared the incidence of AEs in LEN-treated lower-risk MDS pts with or without del(5q). The non-del(5q) group included 416 pts from 4 studies: MDS-005 (n = 160), MDS-002 (n = 215), MDS-001 (n = 24), and PK-002 (n = 17). The del(5q) group included 243 pts from 5 studies: MDS-003 (n = 148), MDS-004 (n = 69), MDS-007 (n = 11), MDS-001 (n = 8), and PK-002 (n = 7). A TEAE was defined as an AE that began or worsened in severity on or after the first dose of LEN through to 28 days after the last dose of LEN. Pts received the recommended starting dose of 10 mg LEN for ≥ 1 cycle; in study MDS-005, pts with impaired creatinine clearance (CrCl; ≥ 40 to < 60 mL/min) had a LEN 5 mg starting dose in order to achieve a similar area under the curve as pts with normal CrCl who were receiving LEN 10 mg.
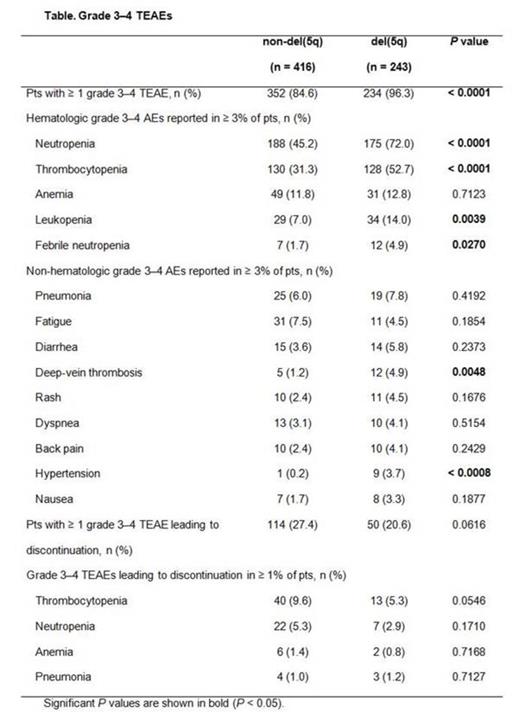
Results: Among the LEN-treated lower-risk MDS pts with or without del(5q) in this pooled analysis, the most commonly reported TEAEs (any grade) occurring in ≥ 5% of pts were hematologic: neutropenia [49.3% vs 73.7% for non-del(5q) vs del(5q), respectively], thrombocytopenia (37.3% vs 64.2%), and anemia (16.8% vs 20.2%). Overall, 84.6% of non-del(5q) pts and 96.3% of del(5q) pts experienced grade 3-4 hematologic TEAEs, including neutropenia [45.2% vs 72.0% for non-del(5q) and del(5q), respectively], thrombocytopenia (31.3% vs 52.7%), and anemia (11.8% vs 12.8%) (Table). Non-hematologic TEAEs were similar for both non-del(5q) and del(5q) pts, except deep-vein thrombosis (1.2% vs 4.9%, respectively) and hypertension (0.2% vs 3.7%). Acute myeloid leukemia was reported as a TEAE in 3 non-del(5q) and 9 del(5q) pts. Bleeding events (any grade) occurring concurrently with grade 3-4 thrombocytopenia were observed in 20.7% of non-del(5q) and 24.4% of del(5q) pts. Infection (any grade) occurring concurrently with grade 3-4 neutropenia was observed in 33.6% of non-del(5q) and 54.0% of del(5q) pts. Analysis of grade 3-4 hematologic TEAEs for pts receiving long-term (> 12 months) LEN treatment by time of onset (0 to 6, > 6 to 12, and > 12 to 18 months) showed that onset rates of grade 3-4 neutropenia during the first 6 months were higher versus rates at > 6 to 12 months for non-del(5q) (42.9% vs 19.5%, respectively) and del(5q) pts (65.4% vs 21.3%). Rates decreased similarly for thrombocytopenia in non-del(5q) (13.0% vs 5.2%) and del(5q) pts (40.4% vs 6.6%). At > 12 to 18 months, onset rates of neutropenia and thrombocytopenia for non-del(5q) pts were 15.6% and 9.1%, respectively; rates for del(5q) pts during this period were 23.5% and 4.4%.
Grade 3-4 TEAEs resulted in discontinuation of LEN in 27.4% of non-del(5q) and 20.6% of del(5q) pts (Table); however, the criteria for discontinuation differed between studies.
Conclusions: In this analysis of pooled data from 7 studies, the safety profiles of LEN-treated lower-risk MDS pts were similar between non-del(5q) and del(5q) pts. Neutropenia and thrombocytopenia were the most common TEAEs in both groups; however, the frequency of these TEAEs was lower in non-del(5q) pts. Among non-del(5q) and del(5q) pts receiving long-term treatment with LEN, onset rates of thrombocytopenia and neutropenia were lower at > 6 to 12 months versus the first 6 months of treatment. In summary, TEAEs in lower-risk MDS pts with or without del(5q) treated with LEN 10 mg for ≥ 1 cycle are predictable, well characterized, and clinically manageable.
Almeida:Shire: Speakers Bureau; Bristol Meyer Squibb: Speakers Bureau; Celgene: Consultancy; Novartis: Consultancy. Off Label Use: Lenalidomide used to treat MDS patients without del(5q). Santini:celgene, Janssen, Novartis, Onconova: Honoraria, Research Funding. Vey:Celgene: Honoraria; Roche: Honoraria; Janssen: Honoraria. Giagounidis:Celgene Corporation: Honoraria. Hellström-Lindberg:Celgene Corporation: Research Funding. Mufti:Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Skikne:Celgene Corporation: Employment, Equity Ownership. Hoenekopp:Celgene International: Employment, Equity Ownership. Séguy:Celgene International: Employment. Zhong:Celgene Corporation: Employment, Equity Ownership. Fenaux:CELGENE: Honoraria, Research Funding; NOVARTIS: Honoraria, Research Funding; AMGEN: Honoraria, Research Funding; JANSSEN: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal