Abstract
Background Polymorphisms in mitochondrial DNA can be used to group individuals into haplogroups that reflect human global migration. These mitochondrial variants are associated with differences in mitochondrial function and have been associated with multiple diseases, including cancer. In this analysis, we evaluated the association between mtDNA haplogroup and risk of myelodysplastic syndromes (MDS).
Methods Cases were identified by rapid case ascertainment through the population-based Minnesota Cancer Surveillance System (MCSS). Participants were recruited to the MDS study if they were diagnosed with MDS between April 1, 2010 and October 31, 2014. Eligibility criteria included residence in Minnesota, age at diagnosis between 20 and 85 years, and ability to understand English or Spanish. Centralized pathology and cytogenetics review were conducted to confirm diagnosis and classify by subtypes. Controls were identified through the Minnesota State driver's license/identification card list. Genomic DNA from cases and controls was collected using Oragene DNA collection kits (DNA Genotek, Ontario, Canada) and extracted via Autopure LS Instrument according to manufacturer's instructions (Qiagen). We genotyped 15 mtSNPs that capture common European mitochondrial haplogroup variation (Mitchell et al Hum Genet 2014; Raby et al J Allergy Clin Immunol 2007) on the Sequenom iPLEX Gold MassArray platform (Sequenom, Inc., San Diego, CA) in the University of Minnesota Genomics Core. Because haplogroup frequencies vary by race and ethnicity, we restricted analyses to non-Hispanic white cases and controls. All statistical analyses were conducted using SAS v.9.3 (SAS Institute, Cary, NC). Odds ratios (OR) and 95% confidence intervals (CI) were calculated. We also evaluated associations by MDS subtype and IPSS-R risk category.
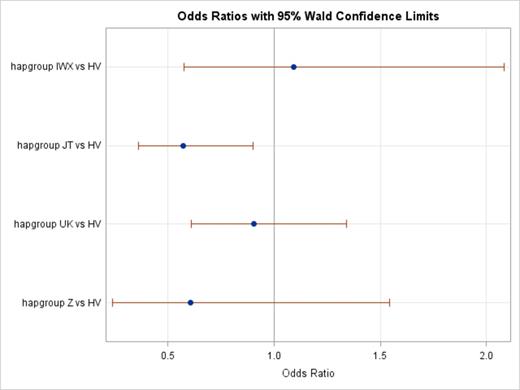
Results We were able to classify 215 cases with confirmed MDS and 522 controls into one of the 11 common European haplogroups. The distribution of haplogroups in our control sample was similar to the distribution reported in a previous sample of non-Hispanic white individuals from the United States (Mitchell et al Hum Genet 2014), with the highest number in the H haplogroup (42%). Due to small sample sizes in some subgroups, we combined mt haplogroups into larger bins based on the haplogroup evolutionary tree, including HV (H+V), JT (J+T), IWX (I+W+X), UK (U+K), and Z (van Oven & Kayser Hum Mut 2009) for comparisons of cases and controls. Using haplogroup HV as the reference group, we found a statistically significant association between haplogroup JT and MDS (OR=0.57, 95% CI 0.36, 0.90, p=0.02). No other significant associations were observed in a comparison of cases and controls (Figure). In the analysis stratified by MDS subtype, the association with haplogroup JT reached statistical significance only in MDS cases with the RCMD subtype (OR=0.42, 95% CI 0.18, 0.97), although the association was similar in magnitude for RARS and the p-value for heterogeneity was non-significant (0.76). Similarly, the associations between haplogroup JT and MDS were similar in the analysis stratified by IPSS-R risk category (p-value for heterogeneity = 0.71).
Conclusions In this population-based study of MDS, we observed an association between mtDNA haplogroup JT and risk of MDS. Previous studies using cybrid cells have reported functional differences by mtDNA haplogroup and provide biological plausibility for the observed association, including higher capacity to cope with oxidative stress in haplogroup T (Meuller et al PLoS One 2012) and lower levels of ATP and reactive oxygen species production in haplogroup J (Kenney et al PLoS One 2013). Further studies of the relationship between mtDNA variation and MDS are warranted in larger sample sizes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal