Abstract
Background:
IUHCT is a non-myeloablative non-immunosuppressive transplant approach that allows for donor cell engraftment across allogeneic barriers. It has the potential to treat a large number of congenital hematologic, genetic, and immunologic disorders. A significant barrier to the clinical application of IUHCT is the low level of donor cell engraftment secondary to competition for limited space in the developing hematopoietic niche. AMD3100, an inhibitor of the chemokine receptor CXCR4, and Firategrast, a small molecule inhibitor of the α4β1 integrin, have been shown to disrupt the retention of hematopoietic stem cells (HSCs) in postnatal bone marrow (BM) transplant models. We hypothesized that administration of these agents to a pregnant dam prior to IUHCT would mobilize endogenous fetal HSCs from the fetal liver (FL) hematopoietic niche resulting in preferential homing of donor HSCs and enhanced long-term donor cell engraftment.
Methods:
AMD3100 (5 mg/kg) and Firategrast (100 mg/kg) were maternally administered alone or in combination to time-dated Balb/c dams at either embryonic day 17 (E17) (pharmacokinetic studies) or E14 (endogenous HSC mobilization, donor cell homing, and long-term engraftment studies). Maternal and fetal sera were analyzed by mass spectrometry at 15, 45, 90, and 180 minutes following AMD3100 or Firategrast administration to determine serum drug concentrations. Based on these pharmacokinetic studies, FLs were harvested 60 minutes following AMD3100 and/or Firategrast administration and assessed by flow cytometry for lineage- sca-1+ c-kit+ (LSK) cells to determine endogenous HSC mobilization. Short-term homing and long-term engraftment were subsequently assessed in Balb/c fetal mice (H2Kd) intravenously injected with 10x106 B6 GFP (H2Kb) BM cells following maternal administration of AMD3100, Firategrast, or AMD3100+Firategrast (Combination). For homing analysis, the proportion of donor LSK within the donor lymphocyte compartment of the FL was measured 24 hours after IUHCT. Long-term engraftment of donor cells was assessed monthly by flow cytometry of peripheral blood (PB) through 6 months of age. Multilineage PB engraftment was assessed at 9 months of age. Statistical analyses were performed using one-way or two-way ANOVA with Bonferroni post-hoc tests.
Results:
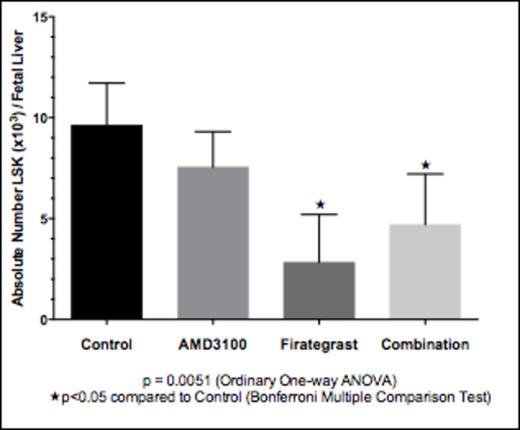
Maternal administration of either agent resulted in transplancental passage with peak fetal serum concentrations occurring between 45 and 90 minutes for AMD3100, and between 15 and 90 minutes for Firategrast. Treatment with Firategrast or Combination (AMD3100+Firategrast) resulted in significant mobilization of endogenous HSCs from the FL, with Firategrast eliciting the greatest mobilization vs. control (no treatment) (2.8x103 vs. 9.6x103 HSCs/FL, p<0.05) (Figure 1). Maternal administration of Firategrast or Combination prior to IUHCT translated into 1) significantly increased short-term homing of allogeneic donor LSK in the fetal liver 24 hours after IUHCT (Control: 1.5% ± 0.10 donor LSK in donor lymphocyte population; Firategrast: 2.4% ± 0.3, p = 0.0135; Combination: 2.4% ± 0.1, p = 0.0136), and 2) sustained enhancement of long-term allogeneic engraftment through 6 months of age (Figure 2). There was no apparent benefit to Combination treatment over Firategrast treatment alone. All chimeric mice demonstrated multilineage engraftment at 9 months of age supporting engraftment at the level of the HSC or early progenitor cell following IUHCT.
Conclusion:
Maternal administration of antagonists of the CXCR4/SDF-1a and a4b1/VCAM-1 pathways results in mobilization of endogenous fetal HSCs from the FL hematopoietic niche and preferential homing of donor HSCs to the FL when administered prior to IUHCT. The combination of agents targeting both the CXCR4/SDF-1a and a4b1/VCAM-1 pathways in the current study did not yield a synergistic effect on mobilization and engraftment. This work demonstrates the potential of mobilizing endogenous fetal HSCs as a strategy to overcome barriers to successful engraftment in utero bringing IUHCT closer to clinical application.
Mobilization of HSCs from fetal liver 1 hour after treatment
Mobilization of HSCs from fetal liver 1 hour after treatment
Treatment enhances long-term allogeneic engraftment through 6 months
Treatment enhances long-term allogeneic engraftment through 6 months
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal