Abstract
Introduction: Allogeneic hematopoietic cell transplantation (HCT) offers a potentially curative therapy in patients (pts) with hematologic malignancies; however, treatment-related mortality (TRM) remains a concern. Strategies to improve post-HCT neutrophil recovery (NR) and immune reconstitution (IR) to decrease TRM need to be explored. Murine models of allogeneic HCT suggest that fractionated hematopoietic progenitor cell (HPC) infusion may improve engraftment through improved access of HPCs to a viable hematopoietic niche (Felfly et al., 2010; Bhattacharya et al., 2009). The impact of fractionated HPC infusion on NR, IR and overall survival (OS) has not been prospectively studied in humans.
Objective: Determine the impact of bulk vs. fractionated HPC infusion on NR after allogeneic HCT, as defined by the time to achieve an absolute neutrophil count (ANC) of > 500. Secondary objectives included evaluations of OS, TRM, relapse and additional parameters of IR.
Methods: In this randomized phase 2 study of bulk vs. fractionated HPC infusions, pts with hematologic malignancies < 75 yrs underwent HCT, receiving unmodified or CD34 selected allografts from HLA-matched or mismatched related or unrelated donors (NCT01596257). Patients were randomized to receive HPC infusion as a bulk (Bulk Arm) or in fractions (Fractionated Arm): 4x10E6 CD34+ cells/kg recipient weight infused on day 0, with the remaining HPCs CD34+ cell selected then infused on days 2, 4 and 6 post-HCT, in equally distributed aliquots. Randomization was stratified by allograft type (unmodified vs. CD34 selected). Patients whose donor failed to collect at least 7x10E6 CD34+ cells/kg recipient weight received bulk HPC infusions regardless of randomization, for safety. These pts continued HCT on study, but were replaced until each arm reached a target accrual of 36 pts. Per protocol, these pts were not included in this modified intent to treat analysis (ITT). Patients with at least 100 days of follow up on 6-30-15 were included in the current analysis.
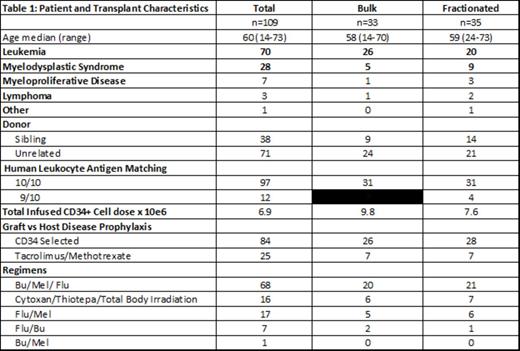
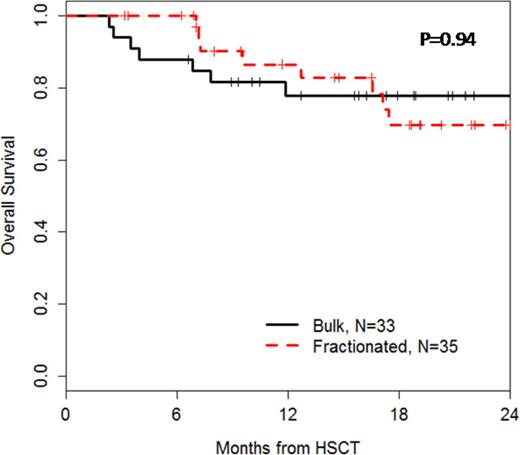
Results: Of 124 pts enrolled between 2012 and 2015, 109 are evaluable. Patient and transplant characteristics are outlined in Table 1. Median age was 60 yrs. Donors of 41 pts (38%) failed to mobilize the minimum CD34+ cell dose and were not included in the analysis. Of 68 pts 33 were randomized to the Bulk Arm and 35 to the Fractionated Arm. Median CD34+ cell doses were 9.8x10E6/kg recipient weight in the Bulk Arm and 7.6x10E6/kg recipient weight in the Fractionated Arm. All pts engrafted. Median time to ANC of > 500 was 11 days in both arms (Figure 1). Day +180 median CD4+ cell count was 173.5 cells/µl in the Bulk Arm and 168 cells/µl in the Fractionated Arm. With a median follow up of 19 months in surviving pts, the estimated 2-yr OS was 78% and 70% for pts in the Bulk vs. Fractionated Arms, respectively (Figure 2). The estimated 2-yr cumulative incidences of TRM were 9% and 7% in pts in the Bulk vs. Fractionated Arms, respectively. The estimated 2-yr cumulative incidences of relapse were 27% and 26% in pts in the Bulk vs. Fractionated Arms, respectively. The cumulative incidences of grades II-IV (grades III-IV) acute GVHD were 30% vs. 14% (6% vs. 0%) in pts in the Bulk vs. Fractionated Arms, respectively.
Conclusion: In this randomized modified (ITT) analysis, fractionated infusion of HPCs in pts undergoing allogeneic HCT did not statistically significantly impact NR or OS when compared to pts receiving bulk HPC infusion. In addition, CD4+ cell count on day 180, TRM and relapse rates were similar between the two groups. We also observed that with current mobilization techniques it was unlikely that more than 60% of normal donors would be able to collect more than 7x10E6 CD34+ cells/kg recipient weight for adult recipients.
KEY-HCT-hematopoietic stem cell transplant, Flu-Fludarabine, Mel-Melphalan, Bu-Busulfan
KEY-HCT-hematopoietic stem cell transplant, Flu-Fludarabine, Mel-Melphalan, Bu-Busulfan
Neutrophil recovery in patients receiving bulk vs. fractionated infusions of hematopoietic progenitor cells
Neutrophil recovery in patients receiving bulk vs. fractionated infusions of hematopoietic progenitor cells
Overall survival in patients receiving bulk vs. fractionated hematopoietic progenitor cell infusions.
Overall survival in patients receiving bulk vs. fractionated hematopoietic progenitor cell infusions.
Off Label Use: We discuss off-label use of busulfan, melphalan, cyclophosphamide, fludarabine, thiotepa, total body irradiation, tacrolimus and methotrexate. We also discuss off-label use of CD34 selected progenitor cell infusion. . Perales:Merck: Honoraria; Amgen: Honoraria; NMDP: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Takeda: Honoraria. Giralt:Celgene: Honoraria, Research Funding; SANOFI: Honoraria; TAKEDA: Honoraria; JAZZ: Honoraria; MILTENYI: Honoraria; Johnson and Johnson: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal