Abstract
Introduction: Combination of proteasome inhibitor (PI) bortezomib and immunomodulatory drugs (IMiDs), lenalidomide or thalidomide, have revealed superior outcomes in multiple myeloma (MM) over their single use. PI and IMiDs possess their direct anti-tumor activity as well as exert the indirect pleiotropic biological effects by modulating immune response, cytokine production, cell adhesion molecule, anti-apoptotic factors, cell cycle regulators. We recently found that circulating immature granulocytes and erythrocytes/megakaryocytes frequently appeared in peripheral blood (PB) in patients treated with bortezomib and IMiDs. In this study, we tested whether this effect is derived from PI, IMiDs or combination, and tried to understand its underlying mechanism to further understand their combinatory effects to normal hematopoiesis.
Patients & Methods: 47 patients aged 37 - 81 years with newly diagnosed or relapsed/refractory MM were enrolled into this study. PB or bone marrow (BM) samples were collected from 7 patients treated with bortezomib/lenalidomide/dexamethasone (VRD), 15 with bortezomib/thalidomide/dexamethasone (VTD), 6 with bortezomib/dexamethasone (BD), 19 with lenalidomide/dexamethasone (RD) regimen. Clonogenic progenitor assay (CFU-C) was performed using PB samples. Change in expression level of adhesion molecules on mobilized immature cells was analyzed with flowcytometric (FCM) analysis. We counted 250 differential WBC in May-Giemsa-stained PB smears under the microscope every other day after start of chemotherapy.
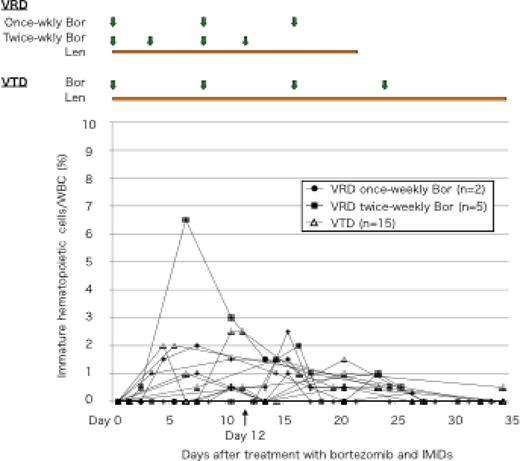
Result: In PB analysis, a significant fraction of immature myeloid or erythro-megakaryocyte cells was morphologically documented in 16 out of 22 (73%) patients treated with VTD/VRD, but never in the patients treated with BD or RD (n=22). During the time course, circulating immature hematopoietic cells gradually increased after the commencement of VRD or VTD therapy, and reached to a peak on a median of 12 days (range, 4 - 24 days), which was documented after 2nd or 3rd administration of bortezomib in the most patients. In these patients, a mean peak percentage of immature cells per WBC was 2.6% (0 - 6.5%) during VRD/VTD therapy. We also performed FCM analyses to confirm mobilization of immature hematopoietic progenitors in peripherally during VRD/VTD therapy. Number of mobilized CD34+ cells was statistically higher in the VRD/VTD group than that in BD/RD group (0.070% vs. 0.017%, p=0.041). We extended an analysis of change in expression of adhesion molecules on mobilized immature cells in PB mononuclear cells, since down-regulation of these molecules resulted in release of stem/progenitor cells from marrow and mobilization into the circulation. We found a significant decrease in mean fluorescence intensity (MFI) of CXCR-4 expressed on PB CD34+ cells in VRD/VTD group compared with that in BD/RD groups (11.1 vs 15.2, p=0.033). Number of CFU-cells (CFU-C) increased after 2nd or 3rd administration of bortezomib during VRD/VTD therapy compared to that before therapy (Figure), which was coincided with the appearance of immature cells in PB. A number of CFU-C derived from 100,000 PB mononuclear cells was increased up to 45 in VRD/VTD group compared to 11 in BD/RD group (p=0.021).
Discussion: In this study, we unexpectedly found a transient mobilization of the immature hematopoietic progenitors in the majority of patients who received concurrently PI and IMiDs, but not in the patients treated with either alone. IMiDs promote degradation of IKZF1, which regulate GATA-1 and PU.1, critical transcription factors for erythroid and granuloid lineage, resulting in a suppression of erythropoiesis and granulocyte maturation arrest with accumulation of immature granulocyte progenitors in the BM. In contrast, PI downregulate expressions such as CXCL-12/CXCR-4 and VLA-4/VCAM-1 by which hematopoietic progenitors anchor to BM microenvironments. Therefore, we hypothesize that accumulated immature progenitors in marrow by treatment with IMiDs, can be quickly released into peripherally by concurrent administration of bortezomib. It is still unknown whether the mechanism of mobilization effect is linked to their anti-myeloma effect. Thus, our observations provided the unexpected biological effects of these drugs. Combinatory use of new drugs should be carefully evaluated to maximize their benefit or prevent possible adverse events.
Akashi:Asahi Kasei: Research Funding, Speakers Bureau; Shionogi: Research Funding, Speakers Bureau; Astellas: Research Funding, Speakers Bureau; Celgene: Research Funding, Speakers Bureau; Chugai: Research Funding, Speakers Bureau; Bristol-Myers Squibb: Research Funding, Speakers Bureau; Novartis Pharma K.K.: Consultancy, Research Funding, Speakers Bureau; Kyowa Hakko Kirin Co., Ltd.: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal