Abstract
Introduction: Total Therapy 4 (TT4) comprises a randomized phase III trial enrolling 289 patients with gene expression profiling defined low-risk MM in which patients were allocated to a standard arm (TT4-S) or a light arm (TT4-L) with as principal goal to reduce toxicity yet maintain efficacy in TT4-L.
Methods:
The TT4-S regimen was similar to TT3b and utilized 2 cycles of VDTPACE induction, tandem transplantation with melphalan 200mg/m2, 2 cycles of dose reduced VDTPACE consolidation and 3 years maintenance with VRD. In TT4-L the number of induction and consolidation cycles was reduced to one each and melphalan was given in a fractionated fashion (50mg/m2/d x 4days) to avoid peak levels of melphalan and reduce mucosal toxicity. Bortezomib and thalidomide were added to the fractionated melphalan conditioning regimen to explore synergistic effects and compensate for potential loss of efficacy.
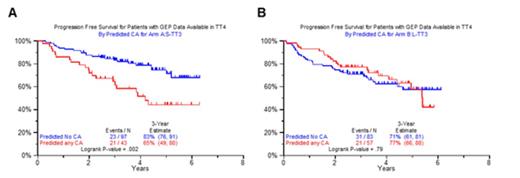
Results: Grade ≥3 toxicities in TT4-S and L occurred with similar frequencies. With a median follow-up of 4.5 years, the OS and PFS were similar in TT4-S and TT4-L at 90 and 87% respectively. The same applied to PFS (TT4-S 84% versus TT4-L 79%). The presence of metaphase defined cytogenetic abnormalities (CA) affected clinical outcomes. In TT4-S, patient with CA had a strong trend toward inferior OS compared to patients with no CA (2 year estimate 83 versus 94%, p=0.08), while the reverse applied to TT4-L (95 versus 81%, p=0.07). Non-significant trends in similar directions were noted for PFS. Complete remission duration tended to be inferior in patients with CA-type MM in TT4-S (2 year estimate, 79 vs. 92%, p=013) with no significant differences in TT4-L. Time to relapse was significantly shorter for CA patients on the TT4-S arm (2 year estimate, 15.4 versus 3.9%), but was not affected by CA in the TT4-L (15.8 vs 7.9%, p=0.79. The observation of CA's favorable OS impact in TT4-L was not anticipated. We next analyzed whether the presence or absence of metaphase CA was linked to specific gene probes which could help to explain better outcomes in TT4-L. Among a training set of 266 untreated patients enrolled in TT3a with available baseline GEP studies, 90 (34%) exhibited CA. Among a test set of 164 patients with baseline GEP accrued to TT3b, 67 (41%) qualified as having CA. Fifty-one probes were different in patients with and without CA (q<0.0001). Seven of the 51 genes had functions in DNA replication, recombination, and repair; five in nucleic acid metabolism, and 4 in RNA post-translational modification and RNA damage and repair. Pathway analysis identified a network of eight interrelated genes that were overexpressed in the CA group, indicating that these MM cells have a higher proliferative activity. We next examined clinical outcomes by the GEP51-CA prediction model in the 2 arms of TT4. In TT4-S, GEP51/no-CA had superior OS and PFS compared to GEP51/CA, which was not observed in TT4-L (Figure 1A, B).
Conclusions: A prognostic CA-linked GEP signature can identify patients who benefit from conditioning with fractionated melphalan dosing together bortezomib, thalidomide and dexamethasone which negates the adverse impact of CA. Patients who lacked a CA-type gene signature were best served with single high dose melphalan. These exploratory findings need to be confirmed in a prospective randomized trial.
PFS according to 51-gene model predicting CA versus no-CA according to arm (TT4-S, 1A; TT4-L, 1B)
PFS according to 51-gene model predicting CA versus no-CA according to arm (TT4-S, 1A; TT4-L, 1B)
van Rhee:University of Arkansa for Medical Sciences: Employment. Mitchell:Cancer Research and Biostatistics: Employment. Zangari:Millennium: Research Funding; Novartis: Research Funding; University of Arkansas for Medical Sciences: Employment; Onyx: Research Funding. Sawyer:University of Arkansas for Medical Sciences: Employment. Waheed:University of Arkansas for Medical Sciences: Employment. Heuck:Millenium: Other: Advisory Board; Janssen: Other: Advisory Board; Celgene: Consultancy; Foundation Medicine: Honoraria; University of Arkansas for Medical Sciences: Employment. Thanendrarajan:University of Arkansas for Medical Sciences: Employment. Schinke:University of Arkansas for Medical Sciences: Employment. Jethava:University of Arkansas for Medical Sciences: Employment. Grazziutti:University of Arkansas for Medical Sciences: Employment. Petty:University of Arkansas for Medical Sciences: Employment. Steward:University of Arkansas for Medical Sciences: Employment. Panozzo:University of Arkansas for Medical Sciences: Employment. Bailey:University of Arkansas for Medical Sciences: Employment. Hoering:Cancer Research and Biostatistics: Employment. Crowley:Cancer Research and Biostatistics: Employment. Davies:University of Arkansas for Medical Sciences: Employment; Celgene: Consultancy; Janssen: Consultancy; Onyx: Consultancy; Millenium: Consultancy. Barlogie:University of Arkansas for Medical Sciences: Employment. Morgan:MMRF: Honoraria; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Weismann Institute: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; CancerNet: Honoraria; University of Arkansas for Medical Sciences: Employment; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal