Abstract
Estimation of band 3 content by EMA fluorescence intensity is rapidly replacing conventional osmotic fragility test as a tool for laboratory confirmation of dominant HS, especially in UK, Europe and certain other countries outside of US (e.g India, Argentina). Its use in US still anecdotal. We have systematically evaluated the utility of EMA test as a screening tool for anemias of various causes and compared the results with those obtained with osmotic gradient ektacytometry-osmoscans, a standard tool in our research laboratory for the last 30 years. Our experience with osmoscans are previously published (Johnson et al, Ravindranath et al) and show distinctive patterns in HS, hereditary elliptocytosis (HE), Southeast Asian Ovalocytosis (SAO), hereditary pyropoikilocytosis (HPP), red cell enzymopathies and hemoglobinopathies. Here we compare the results obtained with osmoscans and EMA testing. An important difference with the two methodologies is that osmoscans measure red cell deformability of all the red cells in the sample across an osmotic gradient from 100-500 mOsmoles (mOsm) while the EMA test reflects the band 3 content of individual red cell. Thus the EMA test is band 3 content as well as cell size dependent. The Omin of osmoscan (lowest osmotic strength at which the cells are still intact and deformable) and the MCF (mean channel of fluorescence) correlate well in dominant HS (usually from mutations in ankyrin, band 3, beta spectrin [SPTB]) in which band 3 is decreased. However, not all samples exhibiting low MCF are due to HS. In fact we observed the lowest MCF values in 3 MCF cases of HPP (MCF 224.4, range 199.7 to 250.8); 34 % reduction of MCF in two Filipino individuals with SAO (319.1, 359.4) and MCF of 390 +/-76 in 19 dominant HS cases versus control values of 514 +/-15, and variably low values in iron deficiency anemia, thalassemia traits, Hb C and cases of autoimmune hemolytic anemias (AIHA). Diagnosis of neonatal HS and distinction form spherocytosis associated with ABO incompatibility remains a challenge. HE cases show distinctive bimodal pattern. A previously unsuspected signature of glycolytic enzyme defects has emerged on scatter plots and histograms with a distinctive tail of small cells - presumably the ATP depleted dense spiculated cells in 3 cases of pyruvate kinase (PK) deficiency and a new case of phosphoglycerte kinase (PGK1) deficiency. In two cases with HS associated with SPTA1 mutations, the MCF was minimally decreased. Representative patterns are shown below.
Conclusions
Thus while all dominant HS cases have low MCF on EMA scan, low MCF per se is not diagnostic of HS. The EMA scan results should be interpreted with caution and both the histograms and dot plots should be analyzed in the context of the clinical picture and morphology; >30% reduction in MCF should raise suspicion of SAO or HPP.
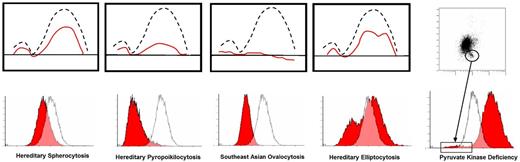
Osmoscan (upper panel) and EMA histogram (lower panel) for HS, HPP, SAO and HE. PKD scatter plot (upper panel) and EMA histogram (lower panel)
Osmoscan (upper panel) and EMA histogram (lower panel) for HS, HPP, SAO and HE. PKD scatter plot (upper panel) and EMA histogram (lower panel)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal