Abstract
Background
Hemophilia A (HA) is caused by a clotting factor VIII (FVIII) deficiency, resulting in spontaneous bleeding and bleeding after minor trauma or surgical procedures. In non-severe HA patients (FVIII ≥ 0.01 IU/mL) a main reason for treatment is surgery. Treatment may consist of FVIII concentrate or desmopressin and other supportive measures. Dosing of FVIII concentrate is currently determined by a patient's weight, baseline FVIII levels and FVIII target values based on the National Hemophilia Consensus. A previous study in severe HA patients (FVIII < 0.05 IU/mL) by Hazendonk et al. ("OPTI-CLOT" studies), showed both under- and overdosing in the perioperative setting. Both may lead to adverse events, such as bleeding in underdosedpatients and a risk of thrombosis and, higher costs in cases of overdosing. However, in non-severe HA patients the perioperative dosing strategy of FVIII concentrates has not yet been systematically evaluated in current practice.
Aim
To evaluate FVIII concentrate treatment perioperatively in non-severe HA patients in the real world and to assess whether under- and overdosing occur.
Methods
This study is a retrospective cohort study. The study population consists of non-severe HA patients in a Dutch hemophilia treatment undergoing surgery between 2008 and 2015 with FVIII concentrate therapy. Data were collected from patient files and included patient and surgical characteristics. To assess the occurrence of under- and overdosing FVIII levels were compared to the FVIII target levels defined based on the National Hemophilia Consensus at each perioperative time point. Preoperatively target peak level was 0.8-1.0 IU/mL. Postoperatively, target trough level was 0.5-0.8 IU/mL for day 1-5 and 0.3-0.5 IU/mL from day 6. Predicting factors for under- and overdosing were determined by using logistic regression, with a significance level of p<0.05.
Results
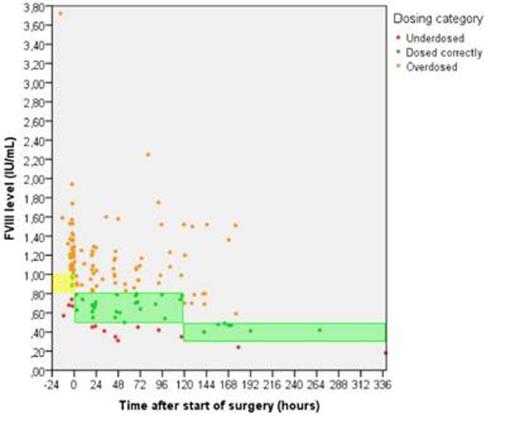
Thirty seven patients (including 2 patients from the study by Hazendonk et al.) underwent 52 surgical procedures in total, of which 5 children undergoing 7 surgeries. Median age was 46 years (range 3-76) and baseline FVIII levels ranged from 0.01 to 0.51 IU/mL (median 0.10 IU/mL) (table 1). Of all 152 perioperative FVIII plasma levels measurements, 14 (9%) were below and 99 (65%) above target FVIII levels. Preoperatively, after bolus infusion of FVIII concentrate, 4/48 (8%) measurements below and 38/48 (79%) above target level. Postoperatively, 104 measurements were evaluated of which 10 (10%) were below target and 61 (59%) above target FVIII levels (see figure 1). Significantly more overdosing was seen preoperatively compared to the postoperative period (p<0.001).
Logistic regression was only possible in the adult subgroup for predicting overdosing and showed that baseline FVIII level is predictive for overdosing as overdosing occurred more frequently in non-severe HA patients with higher baseline FVIII levels (p<0.001). BMI, von Willebrand factor level and blood group were not significant predicting factors in this subgroup.
Conclusion
Overdosing of FVIII concentrates is highly prevalent during perioperative treatment of non-severe HA patients, especially immediately after the preoperative bolus infusion. In adults overdosing occurs more frequently in non-severe HA patients with the higher baseline FVIII levels. This was expected in non-severe hemophilia A patients as also intrinsic FVIII levels increase perioperatively and lead to higher complexity of dosing. These novel data underline the necessity for more patient tailored therapy and alternative future dosing strategies.
demographic data
| . | Study population (n=37) . | Adults only (n=32) . | ||||
|---|---|---|---|---|---|---|
| Age (years) | Mean (sd) | Range | 43,2 (±21) | 3-76 | 48,8 (±16) | 19-76 |
| Weight (kg) | Mean (sd) | Range | 77,5 (±24) | 16-130 | 84,2 (±15) | 57-130 |
| Length (cm) | Mean (sd) | Range | 173,7 (±22) | 102-194 | 179,9 (±7) | 170-194 |
| BMI | Mean (sd) | Range | - | - | 26,5 (±5) | 17-37 |
| Basic FVIII (IU/mL) | Mean (sd) | Range | 0,16 (±0,14) | 0,01-0,51* | 0,16 (±0,15) | 0,01-0,51* |
| VWF antigen (IU/mL) | Mean (sd) | Range | 1,33 (±0,46) | 0,71-2,40 | 1,37 (±0,47) | 0,71-2,40 |
| VWF activity (IU/mL) | Mean (sd) | Range | 1,28 (±0,39) | 0,69-2,11 | 1,31 (±0,40) | 0,69-2,11 |
| Blood group 0 | Number | Percentage | 15 | 42% | 14 | 45% |
| . | Study population (n=37) . | Adults only (n=32) . | ||||
|---|---|---|---|---|---|---|
| Age (years) | Mean (sd) | Range | 43,2 (±21) | 3-76 | 48,8 (±16) | 19-76 |
| Weight (kg) | Mean (sd) | Range | 77,5 (±24) | 16-130 | 84,2 (±15) | 57-130 |
| Length (cm) | Mean (sd) | Range | 173,7 (±22) | 102-194 | 179,9 (±7) | 170-194 |
| BMI | Mean (sd) | Range | - | - | 26,5 (±5) | 17-37 |
| Basic FVIII (IU/mL) | Mean (sd) | Range | 0,16 (±0,14) | 0,01-0,51* | 0,16 (±0,15) | 0,01-0,51* |
| VWF antigen (IU/mL) | Mean (sd) | Range | 1,33 (±0,46) | 0,71-2,40 | 1,37 (±0,47) | 0,71-2,40 |
| VWF activity (IU/mL) | Mean (sd) | Range | 1,28 (±0,39) | 0,69-2,11 | 1,31 (±0,40) | 0,69-2,11 |
| Blood group 0 | Number | Percentage | 15 | 42% | 14 | 45% |
VWF=Von Willebrand factor; sd=standard deviation
*Four patients with baseline FVIII <0.05 IU/mL
measured FVIII levels with FVIII target ranges for;
Yellow bar: preoperative peak levels
Green bars: postoperative trough levels
measured FVIII levels with FVIII target ranges for;
Yellow bar: preoperative peak levels
Green bars: postoperative trough levels
Leebeek:CSL Behring: Other: served on advisory board, Research Funding; Baxter: Consultancy, Other: served on advisory board; Dutch Hemophilia Foundation: Research Funding. Cnossen:Pfizer: Other: travel funding, Research Funding; Baxter: Other: Travel Funding, Research Funding; Bayer: Other: travel funding, Research Funding; CSL Behring: Other: travel funding, Research Funding; Novo Nordisk: Research Funding; Novartis: Research Funding. Kruip:Ferring: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal