Abstract
Introduction
Most coagulation factors are produced by the liver, and therefore in liver cirrhosis both pro- and anticoagulant factor levels are reduced. Cirrhosis patients show no clear systemic bleeding or thrombotic phenotype and thrombin generation (TG) is (almost) unaffected, therefore it has previously been proposed that coagulation is rebalanced in cirrhosis because both pro- and anticoagulant processes are affected to a similar extent. Nevertheless, clinical parameters such as the prothrombin time indicate a bleeding risk in cirrhosis patients, and based on these tests patients often receive blood product transfusion during surgery.
In this study we investigated thrombin generation and its main underlying pro- and anticoagulant processes (prothrombin conversion and thrombin inactivation) to provide empirical evidence for the hypothesis of rebalanced thrombin generation in liver cirrhosis. In addition, we used in silico experimentation to study the consequences of rebalanced TG for current transfusion practices.
Methods
25 cirrhosis patients and 25 healthy subjects were enrolled in our study. The group of cirrhosis patients consisted of 22 Child-Pugh A and 3 Child-Pugh B patients. Prothrombin, antithrombin (AT) and α2Macroglobulin (α2M) levels were determined and TG was measured at 5 pM tissue factor by calibrated automated thrombinography. Prothrombin conversion and thrombin inactivation were quantified from each TG curve, and thrombin-antithrombin and thrombin-α2Macroglobulin complex formation was determined.
The effect of transfusion of prothrombin complex concentrate (PCC) was simulated by an in silico increase of prothrombin conversion. The increase of prothrombin conversion was simulated by substituting the prothrombin conversion curve of a cirrhosis patient by the average healthy subject prothrombin conversion curve, to normalize prothrombin conversion in patients. Thrombin generation curves were calculated based on this normalized prothrombin conversion curve and the original thrombin inactivation parameters of each cirrhosis patient.
Results
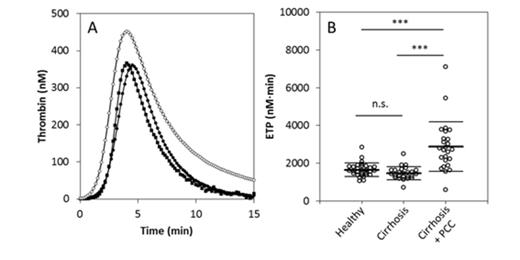
Prothrombin and antithrombin are significantly decreased in cirrhosis patients (74 % and 70 %, p<0.001), whereas the α2M level is significantly increased (p<0.001). Thrombin generation in healthy subjects and patients is similar (figure), although prothrombin conversion (65 %, p<0.001) and thrombin inactivation (73 % p<0.001) are markedly reduced in cirrhosis patients. Acquired AT deficiency results in a reduction of thrombin inactivation by AT (64 %, p<0.001), but the substitution of AT by α2M leads to increased thrombin inactivation by α2M (220 %, p<0.001).
The effect of prothrombin complex concentrate transfusion on thrombin generation in cirrhosis patients was determined by computational modeling (figure). In silico normalization of prothrombin conversion, as would be achieved by PCC transfusion, causes TG to rise to levels that have been shown to be highly prothrombotic (ETP 2878 ± 1323 nM∙min, p<0.001).
Conclusions
Despite large differences in prothrombin conversion and thrombin inactivation, TG in liver cirrhosis patients remains within the normal range (rebalanced), in contrast to the prothrombin time that predicts a bleeding risk in these patients. The transfusion of PCC based on a prolonged prothrombin time would result in a substantial elevation of thrombin generation. This indicates that standard transfusion protocols need to be tailored to the specific needs of cirrhosis patients.
Thrombin generation in healthy subjects (■), cirrhosis patients (●), and cirrhosis patients transfused with PCC (in silico; ○). ***p<0.001
Thrombin generation in healthy subjects (■), cirrhosis patients (●), and cirrhosis patients transfused with PCC (in silico; ○). ***p<0.001
Kremers:Synapse bv: Employment. De Laat:Synapse bv: Employment. Wagenvoord:Synapse bv: Employment. Hemker:Synapse bv: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal