Abstract
Background. Aplastic anemia (AA), the prototypical bone marrow (BM) failure syndrome, is caused by immune-mediated destruction of hematopoietic stem/progenitor cells (HSPCs). CD8+ cytotoxic T cells with restricted TCR diversity (oligoclonal T cells) are expanded in AA, leading to production of proinflammatory cytokines, such as IFN-γ, which induce apoptosis of HSPCs. Recent studies have identified a new subset of memory T cells with stem cell-like properties, TSCM, which are the least differentiated cells of all distinct memory populations. Functionally, TSCM possess an enhanced capacity for self-renewal and can generate multiple memory T cell populations, and they likely have an important role in controlling immunity. In autoimmune diseases, there is abnormal CD4+ and CD8+ T cell activation. We evaluated TSCM frequency in AA and its association with severity, treatment response, relapse, and changes after immunosuppressive therapy (IST). Further, to evaluate the TSCM in other autoimmune diseases, we examined CD4+ and CD8+ TSCM frequencies in uveitis, systemic lupus erythematosus (SLE), and sickle cell disease (SCD), as compared with healthy controls.
Method. We retrospectively analyzed CD4+ and CD8+ TSCM populations by flow cytometry. PB specimens were collected from 55 AA samples and 41 age-matched healthy donor samples. Among 55 AA samples, 21 samples were analyzed at diagnosis and 34 after IST. For comparison, blood samples were obtained from 34 uveitis patients (27 inactive or 7 active cases), 43 SLE patients who met the American College of rheumatology (ACR) criteria for the disease [19 inactive SLE (SLE disease activity index-2K (SLEDAI-2K) score < 3; and 24 active SLE (SLEDAI-2K score > 3)], and 5 SCD patients who were receiving frequent transfusions. TSCM was defined as CD3+ CD4 (CD8)+ CD45RO- CD45RA+ CCR7+ CD27+ CD95+ population.
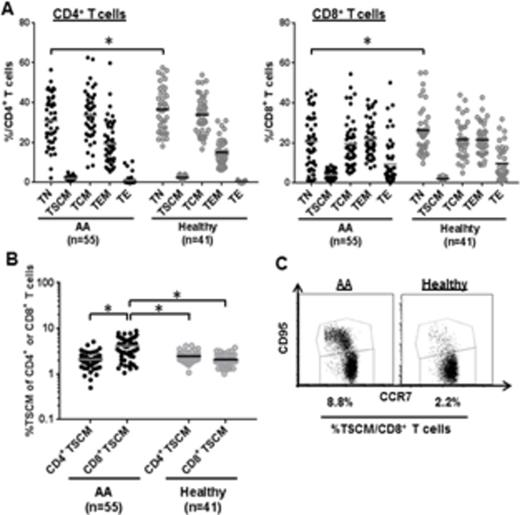
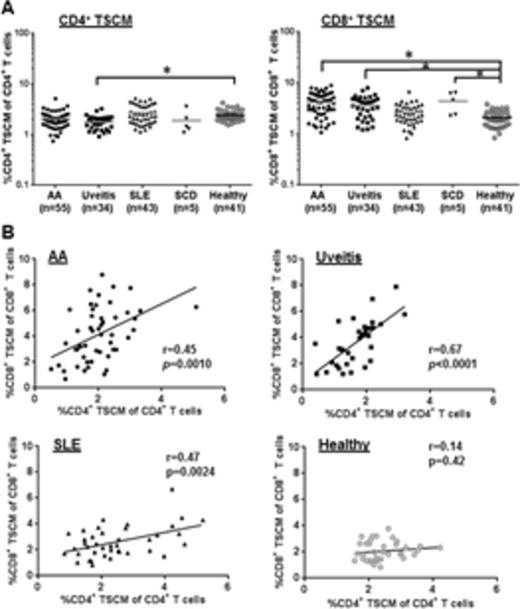
Results and Discussion. In healthy controls, TSCM represented a relatively small percentage of circulating CD4+ or CD8+ T cells (median 2.4% CD4+ TSCM and 2.1% CD8+ TSCM, Fig. 1A). A significantly higher CD8+ TSCM frequency was detected in AA patients (4.2% vs. 2.1%, p < 0.05) while there was no difference in the CD4+ TSCM frequency (p > 0.05), compared to controls (Fig. 1B-C). In AA, high CD8+ TSCM frequency at diagnosis correlated with complete (CR) or partial response (PR) to IST [5.0 % in CR and PR vs 2.8 % in non-responders (NR), p < 0.05). In AA patients prior to IST (n=21), CD8+ TSCM frequency was not correlated with age, sex, absolute neutrophil count, platelet count, time from diagnosis to therapy, and serum ferritin levels. CD8+ TSCM were significantly increased in the two AA cohorts (with or without IST), relative to controls (p < 0.05, respectively). Higher CD8+ TSCM frequency after IST associated with treatment-failure (3.5 % in responders vs 5.5 % in NR or relapse, p < 0.05). Stimulation with anti-CD3/CD28 beads successfully induced cytokine production in CD4+ and CD8+ T cells from AA and healthy controls. Elevated IFN- γ and IL-2 levels were seen in CD4+ and CD8+ TSCM in AA compared to healthy controls. We next compared CD4+ or CD8+ TSCM frequency between each patient group (AA, uveitis, SLE, or SCD) and a healthy control group. Among the four patient groups, the uveitis group alone displayed a reduction in CD4+ TSCM frequency (1.8%) relative to the healthy controls (2.4 %; p < 0.05). An elevated CD8+ TSCM frequency was observed in AA (4.2 %), uveitis (3.6 %), and SCD (4.3 %), but not in SLE, compared to controls (2.1%; p < 0.05) (Fig. 2A). Positive correlation between CD4+ and CD8+ TSCM frequencies was found in AA, autoimmune uveitis, and SLE (Fig. 2B). Evaluation of PD-1 expression revealed that TSCM were the least exhausted T cell compartment, as compared to other types of memory T cells. Immune therapies appeared to have negative effects on the TSCM population both in uveitis and SLE patient cohorts, as well as in AA.
Conclusion. We provide evidence for increased circulating CD8+ TSCM in AA, underscoring the importance of this novel subset in regulation of immune responses and pathogenesis of autoimmunity. Our work described previously unknown potential roles of TSCM in AA, such as cytokine secretion correlated with effector functions. Understanding the CD8+ TSCM population may offer new therapeutic strategies and novel mechanistic insight into the various autoimmune diseases.
Townsley:Novartis: Research Funding; GSK: Research Funding. Dumitriu:Novartis: Research Funding; GSK: Research Funding. Young:Novartis: Research Funding; GSK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal