Abstract
Background: Idelalisib (Zydelig®), a potent and selective PI3Kδ inhibitor, was recently approved for the treatment of relapsed CLL, SLL and FL. PI3Kδ is critical for malignant B-cell proliferation, survival and homing, and inhibition results in a rapid decrease in lymphadenopathy and clinical response (Yang et al., Clin Can Res 2015). Although idelalisib is very potent in mitigating disease, most patients experience incomplete response and ultimately progress. To understand the mechanism of resistance, we screened ABC-DLBCL cell lines for sensitivity to idelalisib and selected TMD8, an ABC-DLBCL cell line, for the generation of idelalisib resistant cells. We report on the mechanisms of resistance and on downstream biomarkers associated with sensitivity to idelalisib in lymphoma cell lines.
Methods: Growth inhibition to idelalisib or other inhibitors was assessed using CellTiterGlo viability assay (Promega) at 96 h. Idelalisib resistant line (TMD8R) was generated by continuous exposure to1μM idelalisib (~2x Cmax corrected for protein binding). A DMSO passage matched line was generated as control (TMD8S). Clonal isolates from pools were generated through 2 rounds of limiting dilution. Cell lines were analyzed by whole exome sequencing (WES, GeneWiz), RNASeq (Expression Analysis) and phosphoproteomics (MultiPathway PTMScan Direct, Cell Signaling Technologies). Protein expression was measured using Simple Western (Protein Simple) and SDS/PAGE Western blot.
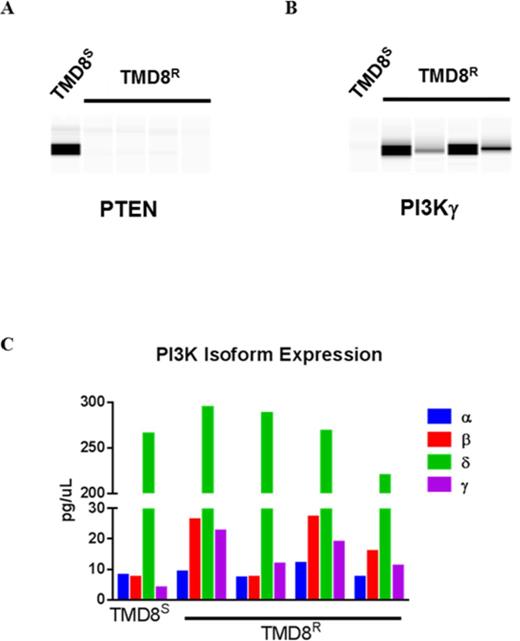
Results: TMD8 were sensitive to idelalisib and the pan PI3K inhibitor (GDC-0941) with an EC50 of 42 and 27nM, respectively, but not to PI3Kα (BYL-719) or PI3Kβ (AZD-6482) inhibitors, indicating that cell viability is mostly driven by PI3Kδ. TMD8 cells with acquired resistance to idelalisib (TMD8R) showed a loss of sensitivity to idelalisib, with a growth inhibition of 19% vs. 92% at 1μM as compared to TMD8S. No mutation in PIK3CD or other PI3K pathway members, including PTEN, was found in TMD8R clones by WES. In 8/8 TMD8R clones a dramatic reduction of PTEN protein expression was observed (9-fold) despite normal expression of PTEN mRNA by RNAseq (Figure 1A). Additionally 8/8 TMD8R clones showed a modest up-regulation of PIK3CG mRNA (2-fold) and protein (3-5 fold, Figure 1B). PIK3CD remained the most prevalent PI3K isoform expressed in all TMD8R clones (Figure 1C). TMD8R were cross-resistant to the dual PI3Kδ/γ inhibitor duvelisib (EC50 > 4 uM for TMD8R vs. EC50 = 58 nM for TMD8S). RNAseq analysis of idelalisib sensitive and resistant ABC-DLBCL cell lines showed that idelalisib treatment led to c-Myc mRNA down regulation in sensitive cell lines (TMD8 and Ri-1) but not in the resistant cell lines (U2932 and SU-DHL-8). In addition, expression of c-Myc target genes was unchanged in the TMDR. Analysis of phosphoproteins showed PI3K and MAPK pathway up-regulation in TMDR, compared to TMD8S, with increased expression of p-Akt T308, p-Akt S473, p-PDK1 S241, p-GSK3β S9 and p-ERK T202/Y204 (11-, 6-, 2-, 1- and 8-fold, respectively), providing a potential mechanism for the loss of c-Myc down regulation in resistant cells (Wen-Bin Tsai et al., Cancer Res, 2012). Other B-cell receptor signaling proteins (p-BTK Y223, p-SYK Y525/526, and p-STAT-5 Y694) were not detected or unchanged. TMD8R cells were cross resistant to the BTK inhibitors ibrutinib and ONO/GS-4059. Akt (MK-2206) or PDK1 (GSK233440) inhibitors were less potent in TMD8R vs. TMD8S cells, yet in combination with idelalisib at 1μM, their potency was restored to the level observed in TMD8S, indicating that resistant cells retain some dependency on the PI3Kδ.
Conclusions: Resistance to idelalisib in the ABC-DLBCL cell line TMD8 was not acquired through de novo mutations. Rather loss of PTEN protein expression, modest up regulation of PI3Kγ, activation of the PI3K and MAPK pathway and loss of c-Myc down regulation by idelalisib were identified as potential mechanism of resistance. Treatment with agents targeting the PI3K pathway in combination with idelalisib may overcome resistance.
Profiling of TMD8R Cells Reveal Increased PI3Kγ and PTEN loss. (A) Decreased expression of PTEN (9 fold) in TMD8R clones as compared to TMD8S (B) Overexpression of PI3Kγ (3-5 fold) in TMD8R clones as compared to TMD8S (C) PI3Kδ isoform is the highest expressed isoform in both TMD8S and TMD8R clones.
Profiling of TMD8R Cells Reveal Increased PI3Kγ and PTEN loss. (A) Decreased expression of PTEN (9 fold) in TMD8R clones as compared to TMD8S (B) Overexpression of PI3Kγ (3-5 fold) in TMD8R clones as compared to TMD8S (C) PI3Kδ isoform is the highest expressed isoform in both TMD8S and TMD8R clones.
Meadows:Gilead Sciences: Employment, Other: Share holder. Rick:Gilead Sciences: Employment, Other: stock holder. Anella:Gilead Sciences: Employment, Other: Stock holder. Liu:Gilead Sciences: Employment, Other: stock holder. Li:Gilead Sciences: Employment, Other: Share holder. Yue:Gilead Sciences: Employment, Other: Share holder. Queva:Gilead Sciences: Other: Stock holder. Tannheimer:Gilead Sciences: Employment, Other: Share holder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal