Abstract
Introduction: The technique of selectively depleting alloreacting T cells from the stem cell transplant (SCT) to prevent graft-versus-host disease (GVHD) while sparing T cells with useful reactivity against viruses and leukemia is commonly termed selective depletion (SD). This approach involves the ex vivo stimulation of donor T cells with host antigen presenting cells (APCs) followed by targeted elimination of the activated alloreactive donor T cell repertoire. SD has been achieved through immunotoxin or magnetic bead depletion targeting T cell activation markers such as CD25 or photodepletion with a rhodamine-like dye. These strategies, while having some success in clinical trials, are limited by poor depletion efficacy or off-target depletion. Here, we present an optimized, novel SD method employing ex vivo treatment of alloactivated T cells with pharmacological concentrations of the purine nucleotide adenosine that preserves quiescent lymphocytes, regulatory T cells (Treg), and T cells with antiviral and antileukemic specificities.
Methods: Mature dendritic cell (DC) or irradiated peripheral blood mononuclear cell (PBMCs) "recipient" stimulators were co-cultured with HLA mismatched "donor" PBMC for up to 7 days. Pharmacologic grade adenosine was added at various time points in culture at doses ranging from 100uM-2mM. Depletion efficacy was analyzed by proliferation of the depleted product when challenged separately against stimulator, responder, and 3rd party APCs in 5 day CFSE-dilution assay to measure residual alloreactivity, background proliferation, and response to de novo antigens. Activity against viral antigens was assessed through 6 hour peptide challenge followed by flow-cytometry based intracellular cytokine staining. Leukemia-specific cells were generated from the allodepleted product through two weekly stimulations with autologous DCs transduced to express leukemia associated antigens WT1 and PRAME.
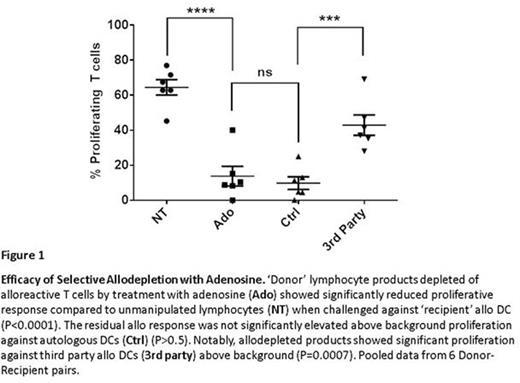
Results: Adenosine reduced alloreactive T lymphocytes in HLA-mismatched allogeneic co-cultures to background control frequencies or lower (Figure 1). Optimum allodepletion was achieved with addition of 2mM adenosine on days 1, 2, and 5 after establishing co-culture. Allodepleted lymphocyte products comprised 37±3% of initial cell numbers with 90±1% viability (n=18). Similarly, this method reproducibly achieved SD in four haploidentical donor-recipient pairs, reducing CFSE-tracked proliferation in challenge against haplo APCs below the background of proliferation in challenge against autologous APCs. Adenosine depletion equally affected CD4 and CD8 T cell subsets while sparing NK and B cell populations. Analysis of naïve, effector memory (EM), central memory (CM), and terminal effector (EMRA) T cell compartments revealed no significant differences in depletion of a particular subset in the allodepleted T cell products (n=6). Critically, CD4+CD25+FOXP3+ Treg populations (n=6) and activity against viral antigens (cytomegalovirus, Epstein-Barr virus, and adenovirus) were maintained and in some cases enriched (n=6). Significant activity against leukemia associated antigens (PRAME and WT1) was achieved in allodepleted products from 3 donors.
Conclusion: This novel SD technique employing adenosine as a pharmacological agent satisfies the requirements of allodepletion with preservation of viral and leukemia-specific immune responses and thus presents a potentially economical method to deplete alloreacting T cells in SCT products for clinical applications.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal