Abstract
Background: Age, prior thrombotic events, and an elevated hematocrit are established risk factors for increased risk of thrombosis in patients with polycythemia vera (PV). Evidence also suggests that elevated white blood cell (WBC) counts ≥ 11×109/L are a significant independent risk factor for thrombosis in patients with PV (P =0.02; Barbui T, et al. Blood. 2015; 126:560-561). Therefore, this analysis was conducted to evaluate the impact of ruxolitinib (Rux) and best available therapy (BAT) treatment on WBC counts among patients with Baseline WBC counts ≥ 11×109/L in the RESPONSE trial. RESPONSE is a global, multicenter, open-label phase 3 trial investigating Rux and BAT in patients with PV who are resistant to or intolerant of hydroxyurea (HU). In the RESPONSE trial, Rux was associated with durable improvements in hematocrit control without phlebotomy compared with BAT as well as reductions in WBC counts.
Methods: Changes from Baseline in WBC counts in the Rux arm (n=110), BAT arm (n=112), and HU subgroup of the BAT arm (n=66) were evaluated as part of an exploratory analysis using the RESPONSE 80-Week analysis dataset. Patient subgroups with Baseline WBC counts ≥ 11×109/L and < 11×109/L were analyzed for changes in WBC counts at Weeks 12, 32, and 80. For patients with Baseline WBC counts ≥ 11×109/L, the proportion of patients who achieved a decrease in WBC counts to ≤ 10×109/L or a ≥50% reduction at Week 12 and Week 32, respectively, was summarized; time to achieve the decrease was analyzed by Kaplan-Meier method.
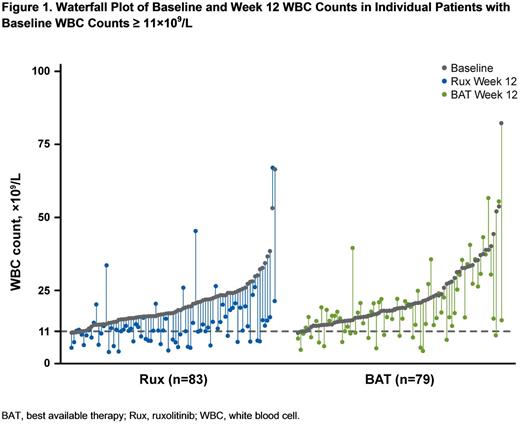
Results: Baseline mean WBC counts were generally similar among patients in the Rux arm, BAT arm, and HU subgroup (17.6×109/L, 19.0×109/L, and 17.4×109/L, respectively). The proportion of patients with Baseline WBC counts ≥ 11×109/L was also similar among the Rux arm, BAT arm, and HU subgroup (75%, 71%, and 70%, respectively). In patients with Baseline WBC counts ≥ 11×109/L, patients treated with Rux had greater mean reductions in WBC counts compared with the BAT arm and HU subgroups, and these reductions were maintained over time; mean changes from Baseline in WBC values at Week 12/Week 32 (×109/L) were -7.7/-7.2 for Rux, -3.2/-4.2 for BAT, and -1.2/-2.2 for HU. In patients with lower Baseline WBC counts, mean values remained stable over time. The change from Baseline to Week 12 for individual patients with Baseline WBC counts ≥ 11×109/L is shown in Figure 1. In patients with Baseline WBC counts ≥ 11×109/L, worsening WBC counts were observed in 10.8% of patients in the Rux arm vs 35.4% in the BAT arm (P =0.0002) and 47.8% in the HU subgroup (P <0.0001). In this same subgroup with elevated WBC counts, a greater proportion of patients in the Rux arm achieved WBC counts ≤ 10×109/L or a ≥50% reduction compared with the BAT arm or HU subgroup (Week 12: Rux, 41% vs BAT, 19% vs HU, 13%; Week 32: Rux, 45% vs BAT, 22% vs HU, 9%); median time to this reduction was 8 weeks in the Rux arm and was not reached in the BAT arm or HU subgroup.
Conclusion: Rux treatment resulted in better control of WBC counts compared to BAT in patients with PV. These changes in the Rux arm occurred early after study initiation (within a median of 8 weeks) and were durable over time. Although RESPONSE was not powered to assess the impact of Rux on thromboembolic events, the lower rate of thromboembolic events observed in the Rux arm vs the BAT arm (1.8 vs 8.2 per 100 patient-years of exposure) is consistent with the observed effects of Rux on hematocrit, WBC counts, and C-reactive protein levels, which are all associated with thromboembolic risk.
Miller:Incyte Corporation: Honoraria, Research Funding. Kiladjian:Novartis: Other: Travel grant; Research Funding paid to institution (Hôpital Saint-Louis et Université Paris Diderot); Incyte Corporation: Consultancy; Novartis: Consultancy. Naim:Incyte Corporation: Employment, Equity Ownership. Sun:Incyte Corporation: Employment, Equity Ownership. Gadbaw:Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Vannucchi:Baxalta: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Shire: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal