Abstract
Background: Patients with polycythemia vera (PV) can have a pronounced symptom burden that negatively impacts their quality of life (QoL). Traditional treatment options such as phlebotomy, hydroxyurea, and interferon provide clinical benefit but frequently do not alleviate symptoms. The ongoing REVEAL study is being conducted to describe contemporary demographics, burden of disease, clinical management, patient-reported outcomes, and healthcare resource utilization among patients with PV in the US. This preliminary analysis describes the burden of PV based on patient-reported outcomes (PROs), including health-related QoL and work productivity.
Methods: REVEAL is a multicenter, noninterventional, nonrandomized, prospective, observational study. Eligible patients are ≥18 years of age with a PV diagnosis and currently under active management by a physician in the US. PROs are being collected at study enrollment and every 3 months thereafter. Each of the 10 items on the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) are graded from 0 (absent) to 10 (worst imaginable), with individual symptom scores ≥7 considered severe; total symptom score (TSS) ranges from 0-100. Raw scores for items on the 30-item European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire-Core 30 (EORTC QLQ-C30) are standardized by linear transformation to 0-100. Higher scores on the EORTC QLQ-C30 indicate better functioning for global health status/QoL and functional subscales and worse severity for symptom subscales. Outcomes with the 6-item Work Productivity and Activity Impairment Specific Health Problem questionnaire (WPAI-SHP) are presented as a proportion from 0% (minimal/no impairment) to 100% (maximal impairment/productivity loss). Analyses of these preliminary data were descriptive.
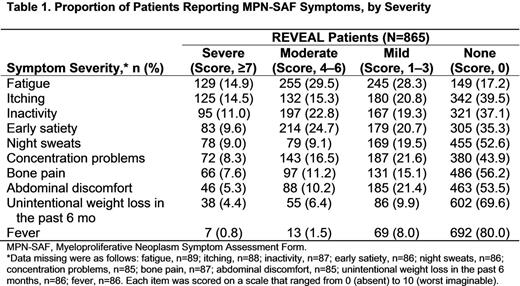
Results: At the time of data cutoff (June 9, 2015), REVEAL had enrolled 865 patients (total planned enrollment, n=2000), the majority were male (55.5%) and white (89.5%), with a median age of 67 (range, 22-95) years. 782 patients had completed the PRO questionnaires at the enrollment visit. A majority of patients reported ≥1 PV-related symptom. Mean (SD) MPN-SAF symptom scores were highest for fatigue (3.5 [2.7]), early satiety (2.7 [2.8]), inactivity (2.7 [2.9]), itching (2.6 [3.1]), and concentration problems (2.1 [2.6]). The mean (SD) MPN-SAF TSS (sum of 10 symptom scores) was 19.6 (16.1). The most frequently reported severe symptoms were fatigue, itching, inactivity, early satiety, night sweats, concentration problems, and bone pain (Table 1). Mean (SD) global health status/QoL reported on the EORTC QLQ-C30 was 72.8 (23.4); functional subscales were as follows: cognitive functioning, 80.3 (24.5); emotional functioning, 80.7 (24.0); physical functioning, 83.1 (20.6); role functioning, 84.3 (26.1); and social functioning, 85.0 (25.0). The most severe mean (SD) symptom subscale scores were fatigue (30.7 [28.5]), insomnia (29.5 [34.5]), pain (20.9 [29.3]), and dyspnea (17.3 [28.6]). Among patients who were employed at baseline (n=273), mean (SD) PV-related WPAI-SHP scores were as follows: absenteeism (ie, work time missed), 2.9% (10.6%); presenteeism (ie, impairment at work/reduced on-the-job effectiveness), 10.7% (20.4%); work productivity loss (ie, absenteeism plus presenteeism), 12.0% (22.2%); and activity impairment, 21.0% (28.0%).
Conclusion: Preliminary descriptive data from patients currently enrolled in REVEAL suggest that patients with PV have notable impairment in QoL because of their disease; patient-reported scores on the MPN-SAF and EORTC QLQ-C30 were similar to those reported previously for patient populations with MPNs. Similarly, PV is associated with marked work productivity loss among patients who are currently employed. Subsequent analyses from REVEAL will evaluate changes over time in PROs and investigate correlations with disease progression and treatment. REVEAL can provide novel insights into questions related to the contemporary real-world QoL burden and its impact on PV-related symptoms and work productivity.
Mesa:Pfizer: Research Funding; NS Pharma: Research Funding; Gilead: Research Funding; Genentech: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Incyte Corporation: Research Funding; CTI Biopharma: Research Funding; Promedior: Research Funding. Boccia:Incyte Corporation: Honoraria. Moliterno:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees. Naim:Incyte Corporation: Employment, Equity Ownership. Cordaro:Incyte Corporation: Employment, Equity Ownership. Peng:Incyte Corporation: Employment, Equity Ownership. Sun:Incyte Corporation: Employment, Equity Ownership. Parasuraman:Incyte Corporation: Employment, Equity Ownership. Stein:Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal