Abstract
Background: TP53 mutations have been reported in 5-10% of patients with myelodysplastic syndromes (MDS) with a higher frequency in those who harbor del(5q) and complex cytogenetics. Next generation sequencing (NGS) data in large clinical cohorts has revealed TP53 mutation is a strong independent prognostic factor. Limited studies have shown an association of p53 protein overexpression with TP53 mutation as well as other clinical characteristics, such as blast count, cytogenetics, and mutant TP53 variant allele frequency (VAF). The study aims to validate the association of p53 overexpression by immunohistochemical (IHC) staining with TP53 mutation, and to explore its relationship to clinical outcome and mutation burden in MDS.
Methods: We retrospectively analyzed MDS patients with or without transformation to acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) diagnosed between 7/2013 and 5/2015. Only those cases with available bone marrow (BM) biopsies with good quality (>1.0 cm in length) corresponding to the NGS testing date were included. Five normal BMs were used as controls. IHC was performed according to our institutional standard protocol and modified in accordance with the manufacturer. Nuclear expression of p53 was assessed semi-quantitatively using an IHC score calculated by multiplying IHC stain intensity with percent positivity in hematopoietic cells. Paired t-test was used for numerical parameters. Hazard ratios were generated using the standard cox proportional harzard model. Survival was analyzed by the Kaplan-Meier method and overall survival (OS) was defined as the duration between date of diagnosis and date of death.
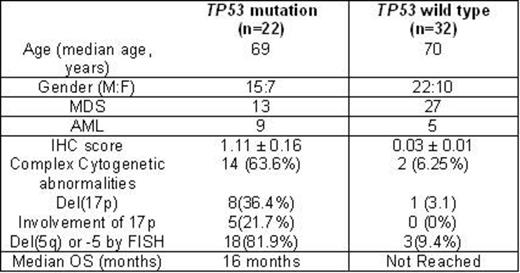
Results: Of 201 patients with myeloid malignancies, 29 (14%) harbored clinically significant mutations by NGS testing. Twenty two of these (9 AML-MRC,13 MDS) had available BM biopsies. An additional 32 patients (27 MDS, 5 AML) with wild type (WT) TP53 were included. Clinicopathologic differences of these groups are summarized in Figure 1. IHC analysis showed significantly (p=1.21x10-6) elevated nuclear p53 expression in TP53 mutated patients (mean score, 1.11± 0.164) compared to WT (0.037 ±0.014). A higher p53 IHC score in mutated patients correlated with a complex karyotype (n=14) compared to those without (n=6) (1.235 ± 0.206, and 0.580 ± 0.257, respectively, p=0.054). A significant positive correlation between IHC score and cytogenetic risk group according to revised international prognostic scoring system ( R-IPSS) existed in our cohort (p<0.001). Importantly, p53 overexpression directly correlated with TP53 VAF within the MDS group (r=.855, p<0 .001). We found no correlation between p53 IHC score and blast count in mutated TP53 patients (r=0.355, p=0.110), but a significant correlation in those with WT TP53 (r=0.527, p=0.002), which merits further investigation. The proportion of del(17p) or del(5q) was greater in the TP53 mutated patients vs. WT patients [40% and 90% in mutated TP53 vs. 4% and 12% in WT, respectively). Median overall survival (OS) was 86 months (95%CI, 7-166) and median follow-up duration was 27 months (95% CI 7-46). The sensitivity and specificity of using a p53 IHC score in predicting TP53 mutation status using a cutoff of 1.00 was 59.1% and 100%, respectively. The sensitivity and specificity of using a p53 IHC score in predicting TP53 mutation status using a cutoff of 0.500 was 77.3% and 100%, respectively. Median OS was shorter in mutated patients [16 months (95% CI, 8-23)] compared to WT (not reached) (p=0.001). OS hazard ratio (HR) of mutant TP53 was 4.6 (95% CI, 1.8-12) (p= 0.002). We also found significant differences in OS by IHC score. Median OS was not reached in patients with an IHC score <1 compared to >1.0 [16 month (95% CI, 8-23)] (p= 0.007). Similarly, with a p53 IHC score cutoff of 0.5, median OS was again not reached in those patients with <0.5 IHC score but was 16 months (95% CI, 8-25) in those >0.5 (p=0.015). HR for IHC score > 0.5 was 3 (95% CI, 1.2-8), p=0.02. HR for IHC score >1.0 was 3.5 [(95% CI, 1.3-9), p=0.01].
Conclusion: p53 overexpression in MDS patients correlates with mutated TP53 and mutation burden. A higher IHC score was associated with poorer clinical features and outcomes. A larger cohort is warranted to define its diagnostic or prognostic utility.
Clinicopathologic Characteristics in Our MDS/AML-MRC Cohort
Clinicopathologic Characteristics in Our MDS/AML-MRC Cohort
Komrokji:Celgene: Consultancy, Research Funding; Pharmacylics: Speakers Bureau; Incyte: Consultancy; Novartis: Research Funding, Speakers Bureau. Padron:Incyte: Research Funding; Novartis: Speakers Bureau. Lancet:Celgene: Consultancy, Research Funding; Amgen: Consultancy; Pfizer: Consultancy; Boehringer-Ingelheim: Consultancy; Kalo-Bios: Consultancy; Seattle Genetics: Consultancy. List:Celgene Corporation: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal