Abstract
Background: The anti-apoptotic protein BCL-2 has been implicated in mediating the survival of multiple myeloma (MM) cells. Venetoclax is a potent, selective, orally bioavailable small-molecule BCL-2 inhibitor. Venetoclax induces cell death in MM cell lines in vitro and primary MM samples ex vivo. Certain genetic subtypes of MM cells are particularly sensitive to venetoclax, including t(11;14) cells, which express a high ratio of BCL2 to MCL1 (venetoclax resistance factor). The current Phase 1 study evaluates safety, efficacy, and pharmacokinetics (PK) in patients (pts) with relapsed/refractory MM.
Methods: Primary objectives are to evaluate safety, PK, and recommended phase two dose; other objectives include assessing preliminary efficacy and the impact of chromosomal abnormalities. In dose-escalation (DE) cohorts, venetoclax was given orally daily at 300, 600, 900, or 1200 mg after a 2-week dose ramp-up (3+3 design). Patients in the safety expansion (SE) cohort received 1200 mg daily after ramp-up. All patients were monitored for tumor lysis syndrome (TLS).
Results: As of June 17, 2015, 37 patients were enrolled in the study: 30 from DE cohorts and 7 from the SE. Median (range) age was 66 years; 19 (51%) were female. Fourteen were ISS stage I, 13 stage II, 8 stage III, 2 unknown. The median (range) number of prior lines of therapy was 6 (1-19). Thirty-two had prior bortezomib (20 refractory), 35 lenalidomide (18 refractory), and 26 had prior stem cell transplant. Fourteen patients had t(11;14), 4 had t(4;14), 5 had del 17p, and 17 had del 13q. Adverse events (AEs) in ≥20% of patients were nausea (49%), diarrhea (38%), vomiting (30%), anemia (27%), fatigue (24%). Grade 3/4 AEs (≥10%): thrombocytopenia (22%), anemia (19%), neutropenia (11 %). Serious AEs (≥2 patients): pyrexia (n=3), cough, malignant neoplasm progression, and sepsis (2 each); 2 (upper abdominal pain and anemia) were possibly related to venetoclax.
Thirty (81%) patients have discontinued venetoclax: 24 due to PD, 3 for AEs (worsening shortness of breath, hypokalemia, and nausea), 2 withdrew consent, 1 due to death (brain hemorrhage following injury). Four deaths occurred (2 due to PD, 1 due to brain hemorrhage, 1 due to pneumopathy). Two of the 6 patients in the 600 mg cohort experienced DLTs of upper abdominal pain and nausea with abdominal pain. No patients met the criteria for laboratory or clinical TLS. Based on preliminary PK (n=21), the mean Cmax and AUC24 were ~dose-proportional at all studied doses (300, 600, 1200 mg) except 900 mg, and dose-normalized venetoclax exposure in MM was similar to that in CLL and NHL pts. Thirty-two of the 37 patients were evaluable for preliminary efficacy (Table).
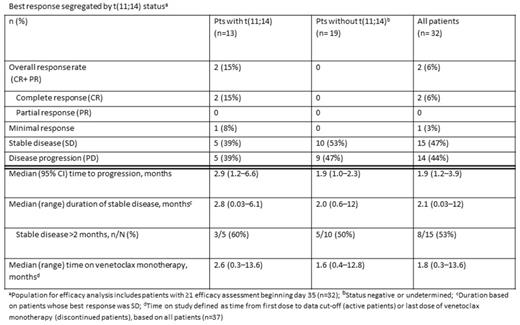
Two patients, both t(11;14), achieved a complete response (1 at 600 mg and 1 at 900 mg). Responses were first achieved at 1.8 and 1.1 months and were maintained for 9.7 and 9.0 months, respectively (900 mg pt remains in CR). Among the 16 patients receiving 1200 mg in the DE or SE cohort, 6 of whom had t(11;14), 5 achieved SD, 6 experienced PD, and 5 are not yet evaluable.
Conclusions: Venetoclax monotherapy had a tolerable safety profile in heavily-pretreated relapsed/refractory MM, and no new safety signals were observed compared to other venetoclax studies. The study continues to enroll in the SE cohort at 1200 mg. Responses (including CR) and longer time on venetoclax were observed in t(11;14) patients. These early results suggest that venetoclax has single agent activity, most prominently in t(11;14) patients.
Kumar:Celgene: Research Funding; Millenium/Takeda: Research Funding; Onyx: Research Funding; AbbVie: Research Funding; Janssen: Research Funding; Sanofi: Research Funding; Celgene, Millenium, Sanofi, Skyline, BMS, Onyx, Noxxon,: Other: Consultant, no compensation,; Skyline, Noxxon: Honoraria. Off Label Use: Venetoclax is an investigational drug that is not yet approved in this indication.. Vij:Takeda, Onyx: Research Funding; Celgene, Onyx, Takeda, Novartis, BMS, Sanofi, Janssen, Merck: Consultancy. Kaufman:Janssen: Consultancy; Spectrum: Consultancy; Merck: Research Funding; Celgene: Consultancy; Onyx: Consultancy; Novartis: Consultancy; Novartis: Research Funding; Onyx: Research Funding. Mikhael:Sanofi: Research Funding; AbbVie: Research Funding; Celgene: Research Funding; Onyx: Research Funding. Moreau:Takeda: Other: Adboard; Janssen: Other: Adboard; Celgene: Other: Adboard; Novartis: Other: Adboard; Amgen: Other: Adboard. Alzate:AbbVie: Employment, Equity Ownership. Morris:AbbVie: Employment, Equity Ownership. Ross:AbbVie: Employment, Equity Ownership. Dunbar:AbbVie: Employment, Equity Ownership. Zhu:AbbVie: Employment, Equity Ownership. Maciag:AbbVie: Employment, Equity Ownership. Agarwal:AbbVie: Employment, Equity Ownership. Leverson:AbbVie: Employment, Equity Ownership. Enschede:AbbVie: Employment, Equity Ownership. Humerickhouse:AbbVie: Employment, Equity Ownership. Touzeau:AbbVie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal