Abstract
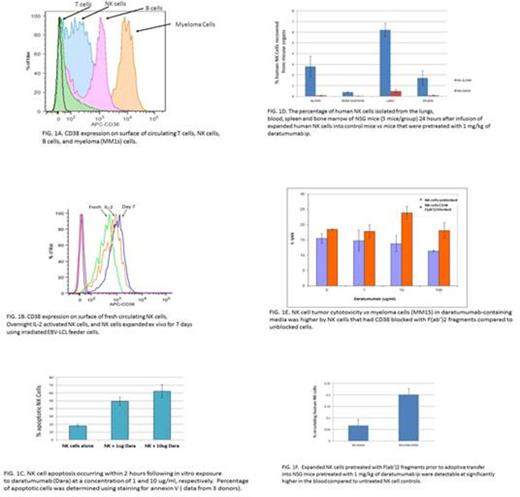
Daratumumab is a fully humanized monoclonal antibody (IgG1) that targets CD38 expressed on myeloma cells. Daratumumab kills myeloma cells through antibody dependent cellular toxicity (ADCC), compliment dependent cytotoxicity (CDC), and antibody dependent phagocytosis (ADCP). In early clinical trials, daratumumab has showed significant anti-myeloma activity in patients with treatment refractory disease. In vivo, daratumumab has been found to induce NK cell lymphopenia of unclear etiology. We found that NK cells isolated from the peripheral blood of healthy and cancer patients expressed variable surface levels of CD38 (Fig. 1A). Further, surface expression of CD38 increased substantially when NK cells underwent ex vivo cytokine activation by culturing cells overnight in IL-2 containing media or ex vivo expansion using irradiated EBV-LCL feeder cells (Fig. 1B). Remarkably, daratumumab induced apoptosis of expanded NK cells in a dose dependent manner, with substantial NK cell apoptosis occurring within 2 hours following in vitro exposure to daratumumab at a concentration of 1 and 10 ug/ml (Fig. 1C). Further, adoptive transfer of ex vivo expanded human NK cells into NSG mice that had been pre-treated with daratumumab showed daratumumab induced NK cell killing in vivo: the numbers of NK cells isolated from the lungs, blood, spleen and bone marrow of NSG mice 24 hours after infusion of expanded human NK cells was reduced by 90% in mice that were pretreated with 1 mg/kg of daratumumab i.p. compared to controls that had not received the antibody (Fig. 1D). In vitro experiments showed NK cell killing by daratumumab occurred as a consequence of ADCC and was dependent on NK cell CD16 expression; when CD56+ NK cells were sorted by FACS into CD16 positive and negative populations, only NK cells expressing CD16 were killed by daratumumab, with no effect on NK cell viability occurring in the CD16- NK cell. Further, we observed that NK cells obtained from donors who have high affinity FCgR3 as a consequence of a single nucleotide polymorphism in the FCGR3A gene resulting in an amino acid substitution at position 158 (F158V) in CD16 were more sensitive to daratumumab killing compared to NK cells isolated from donors carrying the low affinity CD16 polymorphism.
Although NK cell counts and NK reduction in peripheral blood and bone marrow were not associated with daratumumab clinical response in myeloma studies, NK cells play an important role in mediating antitumor responses through ADCC following mAb therapy. In this regard, combining mAb therapy with adoptive transfer of ex vivo expanded NK cells could be utilized as a strategy to potentiate the antitumor effects of mAbs. To overcome daratumumab-mediated killing of adoptively transferred NK cells in daratumumab-treated patients, we blocked CD38 on the surface of NK cells by pretreating them with daratumumab F(ab')2 fragments. The F(ab')2 fragments that were generated using pepsin cleavage of daratumumab were confirmed to bind and block the CD38 epitope expressed on NK cells. Importantly, these F(ab')2 fragments remained bound to the surface of NK cells for at least 96 hours, did not induce NK cell apoptosis, protected NK cells from daratumumab-mediated NK cell killing, and bolstered their tumor cytotoxicity against daratumumab-treated myeloma targets. In vitro experiments showed NK cell tumor cytotoxicity vs myeloma cells in daratumumab-containing media was significantly higher by NK cells that had CD38 blocked with F(ab')2 fragments compared to unblocked controls (Fig. 1E). Importantly, pretreatment with daratumumab F(ab')2 fragments also protected human NK cells from daratumumab-mediated killing in vivo; expanded NK cells pretreated with F(ab')2 fragments prior to adoptive transfer into NSG mice that had been treated with daratumumab were detectable at significantly higher numbers in the blood compared to untreated NK cell controls (Fig. 1F).
Conclusion: Expression of CD38 on activated NK cells makes them susceptible to killing by daratumumab, which could compromise the ability of adoptively transferred NK cells to bolster ADCC following treatment with this mAb. Pretreatment of ex vivo expanded NK cells with daratumumab F(ab')2 fragments protects cells from daratumumab-mediated killing, potentially offering a strategy to augment the anti-tumor effects of adoptively transferred NK cells in myeloma patients that have received daratumumab treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal