Abstract
Introduction: Iron deficiency is one of the most widespread nutritional deficiencies. Globally two billion people are suffering from iron- deficiency anemia (Hermida et al., 2010). Oral therapy for iron deficiency is mainly based on immediate release formulations of ferrous iron. However, modified formulations have been marketed to reduce gastrointestinal side effects and to prevent iron instability in the gastrointestinal tract. Overcoming biological barriers, including the gastrointestinal epithelial barriers, is a great challenge for pharmaceutical research and thus there is a need for new absorption enhancers with more favorable profile. Sucrose esters are widely used in the food industry, and there are reports on their potential use in pharmaceutical formulations as excipients (Szuts A et al., 2008). In vitro methods using cell cultures have been proposed to assess iron bioavailability as an alternative to in vivo methods. Caco-2 cells have shown numerous morphological and biochemical characteristics of enterocytes and have been successfully used to study iron absorption (Garcia et al., 1996; Jovani et al., 2001). Caco-2 monolayers formed a good barrier as reflected by high transepithelial resistance and positive immunostaining for junctional proteins. Sucrose esters in nontoxic concentrations significantly reduced resistance and impedance, and increased permeability of some components in Caco-2 monolayers. Recent data indicate that sucrose esters can enhance drug permeability through both the transcellular and paracellular routes (Kiss et al., 2014).
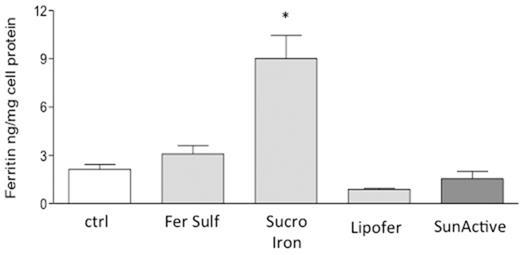
Aim: The strong correlation between the published human absorption data and the iron uptake by Caco-2 cells makes them an ideal in vitro model to study iron bioavailability (Au and Reddy, 2000). For this, in the present study, we compared the bioavailability of innovative Oral Iron formulation based on Sucrosomial Iron¨ (Sideral¨) with three different Iron formulations (Figure 1).
Materials and Methods: Sucrosomial Iron, preparation of ferric pyrophosphate convered by a phospholipids plus sucrose esters of fatty acids matrix; Lipofer¨, a water-dispersible micronised iron; Sunactive¨ ferric pyrophosphate, lecithin and emulsifiers.
Results: The data showed that Sucrosomial Iron¨ (Sideral¨), is significantly more bioavaible than microencapsulated Ferric pyrophosphate ingredients, Lipofer¨ and Sunactive¨ and Ferrous Sulfate in Caco-2 cell model (Figure 1).
Bibliography
Au, A. P., Reddy, M. B. (2000). Caco-2 cells can be used to assess human iron bioavailability from a semipurified meal. J Nutr 130:1329-1334.
Garcia et al. (1996). The Caco-2 cell culture system can be used as a model to study food iron availability. J Nutr 126:251-258.
Hermida et al., Preparation and characterization of iron-containing liposomes: their effect on soluble iron uptake by Caco-2 cells Journal of Liposome Research, 2010, 1-10,
Jovani et al. (2001) Calcium, iron, and zinc uptake from digests of infant formulas by Caco-2 cells. J Agric Food Chem 49:3480-3485.
Kiss et al., (2014) Sucrose esters increase drug penetration, but do not inhibit p-glycoprotein in caco-2 intestinal epithelial cells J Pharm Sci. Oct;103(10):3107-19.
Szuts A et al. (2008) Study of the effects of drugs on the structures of sucrose esters and the effects of solid-state interactions on drug release J Pharm Biomed Anal. 48:
the graph shows the Ferritin levels of Caco-2 cells after iron formulations treatment. Sucrosomial Iron treated cells display significant increase of Ferritin synthesis compared to Lipofer and SunActive treated cells.
the graph shows the Ferritin levels of Caco-2 cells after iron formulations treatment. Sucrosomial Iron treated cells display significant increase of Ferritin synthesis compared to Lipofer and SunActive treated cells.
Tarantino:Pharmanutra s.p.a.: Employment. Brilli:Pharmanutra s.p.a.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal